H2O2 / Plasma: Big Lies, Little Chambers
Dear sterilization solution shopper,
Selecting the best sterilization method for your practice is a critical decision. It affects your medical equipment investment, staff safety, budget and time.
We pride ourselves on providing FACTS backed by scientific data to better arm you to make the best decision for your practice.
Unfortunately, some of our competitors in the hydrogen peroxide sterilization market do not uphold the same values and principles and have been spreading false information to simply “make the sale.”
Below in red are statements made by H2O2 salespeople to prospective customers regarding EO gas sterilization vs plasma. We address and debunk each with facts and resources so you can know the truth about both modalities before making your critical sterilization decision.
Quick note: Hydrogen Peroxide, H2O2 and Plasma are all different names for the same sterilant.

No. EO is not being banned, nor is it currently banned in certain areas. EO continues to be broadly used for processing food products, cosmetics, manufacturing and medical devices. It is a critical sterilant widely used to keep medical devices, implants, endoscopes, (and many more items) safe.
In fact, more than 20 billion devices (approximately 50%) sold in the U.S. annually are sterilized with EO. In many cases EO is the ONLY way these devices can be sterilized because of its highly compatible and gentle nature.
During the deadly 2015 carbapenem-resistant Enterobacteriaceae (CRE) outbreak, the FDA recommended four supplemental reprocessing measures, which included EO sterilization as the most effective in assuring the complete inactivation of highly resistant organisms. (More…)
“FDA is trying to get rid of EO.”
We are very proud of our working relationships with FDA and many other regulatory agencies. Andersen has 18 FDA clearances (all awarded since 2015) and six regulatory marks and approvals for our innovative line of sterilizers.
- We hold the first and only FDA clearance for terminal sterilization of duodenoscopes – up to 11.6 feet long and down to 1.2 mm diameter (that’s very long and very narrow).
- Andersen recently won two national awards for our proprietary ultra-low emission technology. One of them was the FDA Innovation Challenge.
- Andersen representatives sit on several FDA committees and there is no talk of “getting rid” of EO.
“Hydrogen peroxide (H2O2) is very safe. It’s probably under your sink at home!”
Generally, household H2O2 (in that brown bottle) is 3% concentration,1 while sterilization H2O2 is at least 50% concentration. Some sterilizers concentrate it to over 90%.2 For comparison: Rocket fuel is 80-90%.3
A recent report chronicling a scholarly search through FDA’s “MAUD” adverse event database for harm caused by H2O2 sterilizers revealed many reports describing several types of injuries.
“Ethylene oxide is super toxic, h2O2 / Plasma is not.”
No. That is untrue. Anytime a sales representative tells you their sterilant is not toxic, you should immediately be concerned (how, then, is it killing microbes?). Below is a comparative toxicity table containing industry regulatory toxicity limits for both sterilants. As you can see, the sterilants rate very similarly.
| Ethylene Oxide | Hydrogen Peroxide | |
|---|---|---|
| OSHA 8hr/15min PEL: Permissible Exposure Limits | 1 ppm / 5 ppm | 1 ppm / – |
| ACGIH 8hr/15min TLV: Threshold Limit Values | 1 ppm / – | 1 ppm / – |
| NIOSH 8hr/10min REL: Recommended Exposure Limit | <0.1 ppm / 5 ppm | 1 ppm / – |
| NIOSH IDLH: Immediately Dangerous to Life or Health | 800 ppm | 75 ppm |
| HSE 8hr/15min WEL: Workplace Exposure Limits | 1 ppm / – | 1 ppm / 2 ppm |
| CAMEO Datasheet | Ethylene Oxide | Hydrogen Peroxide |
The IDLH and WEL (in red) have more stringent limits for H2O2 than for EO – in the case of IDLH, it’s 10x lower for EO. Pressure is mounting from many sides to set the as-yet undetermined Short Term Exposure Limits or STEL (–) for H2O2.
“The industry is moving towards plasma. EO is an old technology.”
“Plasma” the word itself sounds cutting edge and new. But it’s not. It’s just another word for hydrogen peroxide. Definition: Plasma is what happens when H2O2 gas under deep vacuum is hit with radio frequency or microwave energy.4
The first H2O2 sterilizer hit the market in 1993 – making plasma nearly 30 years old – a “new” version came out in 1998.4
It is far from true that EO technology has stagnated. Andersen received its most recent 510k FDA clearance for a new sterilizer in 2020. As mentioned above we have 18 recent FDA clearances, the FDA Innovation Challenge Award and another recent national award for our proprietary technology.
“H2O2 / Plasma is eco-friendly and safe because it turns into water and oxygen.”
Ask your H2O2 rep where and when their sterilizer “cracks” – or breaks H2O2 down. It is often outside the cabinet. If it is inside the cabinet, it restricts chamber space and the chemicals are often not fully broken down by the time you open the door – exposing operators to amounts far above OSHA levels.
There are some serious arguments against H2O2 being “green” at all.
Also, if “green” status is awarded simply for a sterilant breaking down into something harmless, EO could also qualify. EPA says, “Ethylene oxide reacts in the air to form formic acid, which is a naturally occurring chemical.” And, as Nature.com asserts, “Formic Acid is one of the simplest and most abundant organic molecules in Earth’s atmosphere…”
Does that feel disingenuous to you? Of course it does! While both sterilants break down into harmless elements, what everyone is worried about is how toxic each chemical is before it breaks down – when operators are potentially exposed to it.
Instead of pretending our sterilant isn’t dangerous, we put many redundancies and safety measures in place. It’s all about smart design and stellar training and service. We’d love to talk to you about this in full detail.
“With H2O2 / Plasma, you don’t need to test for operator exposure.”
Yes, you absolutely do. All chemicals capable of achieving terminal sterility are inherently dangerous. Under the OSHA General Duty Clause, employers have an obligation to protect workers from serious and recognized workplace hazards even where there is no standard yet set.
One of the hallmarks of H2O2 / Plasma is that it corrodes the sterilizer and creates malfunctions leading to leaks. There is ample evidence operator exposure at unsafe levels happens frequently. This list is by no means exhaustive.
- The recent report mentioned above chronicling a scholarly search through FDA’s “MAUD” adverse event database for injuries caused by H2O2 sterilizers revealed: skin burns, respiratory complaints, headaches and eye irritation – among other types of harms.
- Anecdotal reports from a ChemDAQ customer showed H2O2 levels of 20-40 ppm at the end of each cycle (keeping in mind that one tech may unload 4 every hour).
- Curious about what the two largest H2O2 sterilizer manufacturers have to say about one another? STERIS circulated this report on STERRAD and then STERRAD responded with this report on STERIS. Both reports document unsafe levels of off-gassing.
- A 2012 IDI report analyzed H2O2 levels around four H2O2 sterilizer models on the market and found the levels were “much higher than expected.” One generally reached around 80 ppm when the door was opened at the end of the cycle and occasionally up to 140 ppm.
So, if you do choose H2O2, how will you monitor for exposure?
Andersen offers easy-to-use personal monitor badges and recommends they be used on installation, once a year and anytime the sterilizer is moved or reinstalled.
“H2O2 is a far more efficient sterilization method.”
Is it? It is faster for sure. But let’s look at that math. If you use it at top efficiency, your staff will need to fill and empty it about 20 times a day (every 32-48 minutes, depending on the sterilizer). Usable chamber space is often quite small.
Keep in mind this point (emptied 20 times a day) and the point above regarding operator exposure upon opening the door: If one tech is “baby-sitting” the sterilizer, that could mean unsafe exposure levels 20 times in one day!
With an Andersen sterilizer, there’s no need for your techs to run back and forth all day, feeling like they’re trying to empty the ocean with a spoon. We have several options to accommodate your work flow: easy one-and-done, fast or many independent loads in one sterilizer. Also, you don’t need to hope no one is being exposed to unsafe levels of a sterilant with Andersen. You can just test for it.
“H2O2 Safely sterilizes instruments without leaving toxic residue.”
You will see claims of zero residuals on most H2O2 sterilizer websites and literature. Sometimes within a few pages of a statement like, “If white residue is visible on the load, this may be hydrogen peroxide residue. The load will need to be reprocessed to prevent potential injury to the patient.”
That same 2012 report mentioned above chronicles the results when researchers analyzed H2O2 residuals on panels made from 11 different types of plastic, on plastic skin staplers and on mock cystonephrofibersopes and bronchofiberscopes.
- 5 of 11 plastic panels showed residuals over 100 ppm immediately after sterilization.
- The mock flexible fiberscopes showed more than 10 ppm for 18 – 40 hours.
- The plastic skin staplers started at 300 ppm, took 24 days to reach 1 ppm and 44 days to reach 0.1 ppm.
Quick reminder: NIOSH set Immediately Dangerous to Life or Health (IDLH) at 75 ppm. And OSHA‘s 8 hour Permissible Exposure Limit (PEL) is 1 ppm.

What we want you to know about
H2O2 & EO …
“All sterilization methods have pros and cons. Let’s talk Honestly about them!”
At Andersen we strive to be a resource for you; the sterilization expert on your team. Your salesperson becomes your account representative and operator trainer. You get free training for the life of your sterilizer and emergency support available 24/7/365. In short: We’re here for you in a very literal sense.
We would love to chat about your needs, all your sterilization options and what will ultimately meet your goals.
There are pros and cons for every method and we absolutely encourage honest and open discussion. To help get that conversation started, we’ve created a side-by-side comparison chart for both modalities.
In the table below, the far left column shows which, in our opinion, “wins” on each point – H2O2 / plasma in red or EO (Andersen) in teal. As previously discussed, it’s pretty much a draw on toxicity so neither side got it.
| H2O2 | EO (Andersen) | |
|---|---|---|
| Speed | Offer the shortest sterilization cycle available ~32-48 min. | Andersen sterilizers’ exposure cycle times range from 3-24 hours |
| Aeration | Do not require long aeration periods after sterilization | .5-24 hours of aeration required depending on the machine/item IFUs. |
| Toxicity | In the table above, the red numbers draw attention to stricter limits for H2O2 over EO. | In the table above, the blue numbers are those that favor EO or are the same as those given to H2O2. |
| Acquisition cost | You pay for speed. Hydrogen peroxide sterilizers tend to be the most expensive option for healthcare facilities. | Andersen sterilizers use ethylene oxide flexible chamber technology (EO-FCT) which is a less mechanically complex and affordable technology. |
| Labor / Consumable costs | Have relatively small chambers, which require an operator to run numerous cycles throughout the day. This results in higher consumable and labor costs. | Andersen sterilizers have a much larger chamber and are typically run overnight and over the weekend. The sterilizer is working while you are sleeping and having fun. |
| Service cost | Hydrogen peroxide sterilizers have expensive maintenance contracts that are usually contractually required for continued operation. They are expensive to maintain because of the corrosive nature of the sterilant. | EO is non-corrosive and causes no harm to the machine. Regular preventative maintenance is both affordable and optional. Customers continually rave about the longevity and reliability of our serilizers and their low maintenance needs. |
| Compatibility | Not compatible cloth, paper, liquids, powders, any materials containing cellulose.5 Hydrogen peroxide is corrosive and is not compatible with anodized aluminum. Repeated sterilization of plastic can cause it to become brittle and crazed. It is not compatible with oxidizable materials (iron, copper, brass, bronze, chromium, zinc, lead, silver, manganese). [Note: Contact with combustible material may result in SPONTANEOUS combustion.]6 | The most widely used low temperature sterilant, and it is still the dominant process used by the medical device industry, owing to its effectiveness and compatibility with most materials. Andersen sterilizers offer the most delicate EO cycle available and are broadly compatible with a wide range of materials (including cloth, paper and cellulose) and packaging options. |
| Long Lumens | Most have severe limitations for devices with long and/or narrow channels or lumens.5 This includes scopes and tubing. | Andersen sterilizers offer excellent penetration and have cleared by FDA for the sterilization of channels down to 1.2mm in diameter and over 11’ in length! |
| Deep Vacuum | Pull a very deep vacuum at the start of the cycle that can damage many devices. | No deep vacuum. Andersen sterilizers can be used to sterilize delicate electronics and other materials that are incompatible with hydrogen peroxide. |
| Reliability | The powerful pumps and corrosive nature of hydrogen peroxide introduce many possible failure points. Malfunctions and cycle aborts can result in hydrogen peroxide being released into the workplace. | Andersen sterilizers are designed with a “chamber inside of a chamber” – which provides a redundancy to protect the work environment from chemical exposure. |
“compare both cabinet size and chamber size, as you consider options!”
One easy-to-see difference, is H2O2 / Plasma sterilizers tend to have a large footprint and relatively small chambers. In comparison our sterilizers fit on a counter or a table and the chamber takes up the vast majority of the machine, give you maximum flexibility.
All measurements below are for the chambers (other than the background 5’4″ line).
large footprint, small chamber
Small footprint, Large chamber
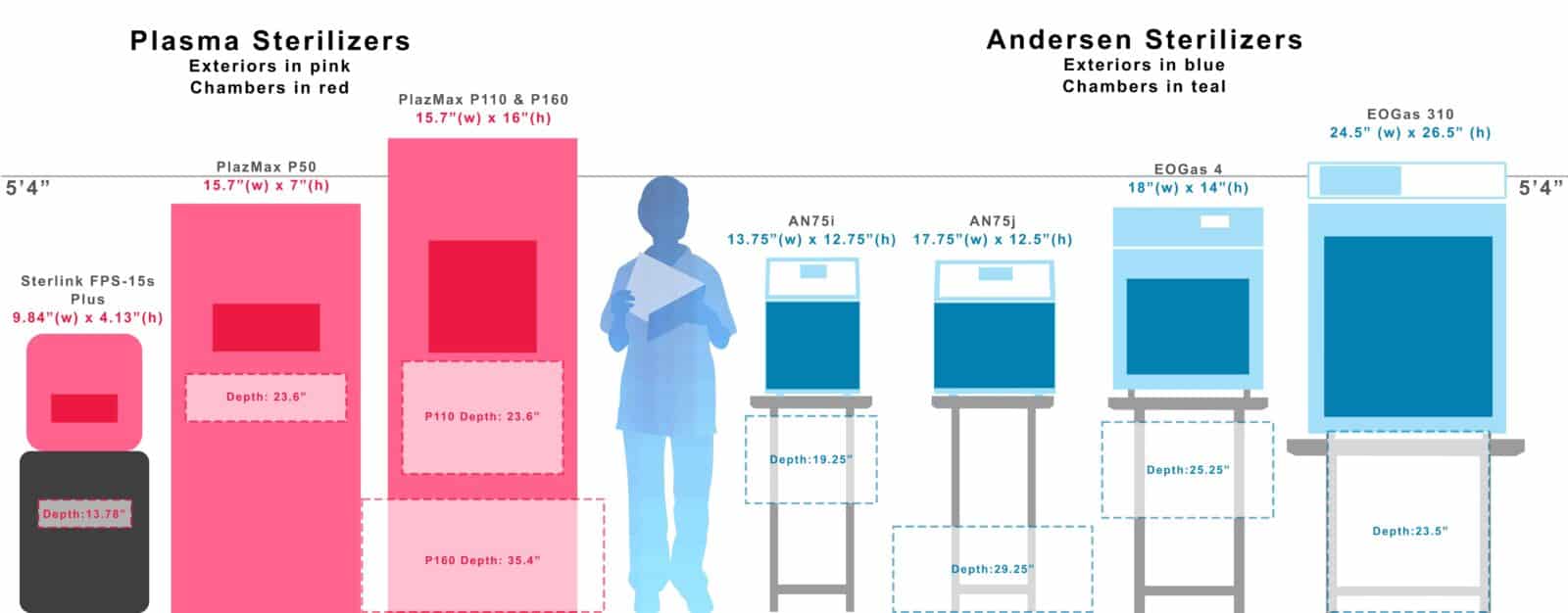
“Do your Research! be sure to weigh all the variables before you buy.”
Below is a detailed comparison table of available H2O2 / Plasma sterilization options in the veterinary market against Andersen sterilizers. Each sterilizer name provides a link to its company-owned product information webpage or user manual so you can do further research on your own. Also, when we say “functional capacity” we mean the actual area you can fill with items to be sterilized.
detailed Sterilizer Comparison: H2O2 vs. Andersen Sterilizers
| Plasmapp Sterlink FPS-15s Plus | Tuttnauer PlazMax P50 | Tuttnauer PlazMax P110 | Tuttnauer PlazMax P160 | Andersen Anprolene AN75i | Andersen Anprolene AN75j | Andersen EOGas 4 | |
|---|---|---|---|---|---|---|---|
| Sterilant | 59% H2O2 Vapor | 50% H2O2 Vapor | 50% H2O2 Vapor | 50% H2O2 Vapor | 100% EO | 100% EO | 100% EO |
| Sterilant dose (ml=g) | Not disclosed | 10ml – 12.5 ml / cycle | 15 ml – 18.75 ml | 25 ml – 37.5 ml | 17.6 g | 17.6 g | 17.6 g |
| Delivery | Cassette | Bottle | Bottle | Bottle | Cartridge | Cartridge | Cartridge |
| Sterility Assurance Level (SAL) Claim | 10-6 | NO claim | NO claim | NO claim | 10-6 | 10-6 | 10-6 |
| Stated capacity | 14 Liters | 47 Liters | 109 Liters | 162 Liters | 35 Liters | Same | 35 Liters |
| Functional capacity | 9.84″ (w) x 13.78″ (d) x 4.13″ (h) – largest tray = 9.18 Liters | (1) Tray Dimensions: 15.7″ (w) x 23.6″ (d) x <7″ (h) = 42.5 Liters | (2) Tray Dimensions: 15.7″ (w) x 23.6″(d) x <8″ (h) = 97.2 Liters | (2) Tray Dimensions: 15.7″ (w) x 35.4″(d) x <8″ (h) = 145.8 Liters | Usable bag dimensions: 22″ (w) x 30″ (d) x 6″ (h) = 35 Liters | Same | Usable bag dimensions: 22″ (w) x 30″ (d) x 6″ (h) = 35 Liters |
| Cycle temperature | 56°C | 55°C | 55°C | 55°C | Room Temp (20° – 33°C) | Same | 50°C or 30°C |
| Exposure cycle time | 7-36 min | 32-40 min | 37 – 44 min | 41 – 48 min. | 12 or 24 hours | Same | Exposure & Aeration: 3.5 or 5 hours |
| Aeration | None | None | None | None | 2, 12 or 24 hours | Same | Included above |
| Pressure/vacuum | 4.1 mBar (99.6% vacuum) = 3.9 lbs of force | 0.1 mBar (>99.9% vacuum) | 0.1 mBar (>99.9% vacuum) | 0.1 mBar (>99.9% vacuum) | 14.9 mBar (~98.5% vacuum) | 14.9 mBar | 14.9 mBar |
| Exterior dimensions | 17.04″ (w) x 24.17″ (d) x 17.20″ (h) | 27.6″ (w) x 60.1″ (h) x 28.7 ” (d) / or 28.9″ w 2 doors | 27.6″ (w) x 69.6″ (h) x 28.7 ” (d)/or 28.9 w 2 doors | 27.6″ (w) x 69.6″ (h) x 40.5″ (d)/or 40.7″ w 2 doors | 14″ (w) x 21″ (d) x 20.5″ (h) | 18″ (w) x 31.5″ (d) x 20″ (h) | 22″ (w) x 29.5″ (d) x 28″ (h) |
| Plasmapp Sterlink FPS-15s Plus | Tuttnauer PlazMax P50 | Tuttnauer PlazMax P110 | Tuttnauer PlazMax P160 | Andersen Anprolene AN75i | Andersen Anprolene AN75j | Andersen EOGas 4 | |
|---|---|---|---|---|---|---|---|
| FDA-Cleared | Yes, 2021, 36 min cycle ONLY | No | No | No | Yes, 2017 12 hour cycle | Yes, 2017 12 hour cycle | Yes, 2015, 3.5 hour cycle |
| Cleared for | Instruments with diffusion-restricted spaces and medical devices with a single stainless-steel lumen with inside diameter of >2.4 mm and a length of <280 mm. No more than 5 and weighing <3.97 lbs. | N/A | N/A | N/A | Metal, 24 lbs Plastic, 3.5 lbs Fabric, 3 lbs Cellulose and Tyvek also included by way of wrapping clearances. Includes hinged or mated surfaces. | Same | Metal, 24 lbs Plastic, 7 lbs Fabric, 6.1 lbs Single-lumen Endoscopes: (1) >2 mm diameter, 1100 mm length Or up to (4) >1.2 mm diameter, <700 mm length Cellulose, Tyvek as well as hinged or mated surfaces |
| Shelf life of sterilized items | None claimed | None claimed | None claimed | None claimed | At least 3 months (FDA-clearance) | Same | At least 3 months (FDA-clearance) |
| Biological Indicator | Yes, 30 min | Yes, 30 min | Yes, 30 min | Yes, 30 min | Yes, 48 hours | Same | Yes, 48 hours |
| Installation | 110/230v, requires heat sealer | 230v only | 230v only | 230v only | 110/230v, vent to outside | Same | 110/230v, vent to outside |
| Diffusion | Does not say | “Cracks” outside cabinet | “Cracks” outside cabinet | “Cracks” outside cabinet | Smart design pulls all gas to the outside where it quickly breaks down and dissipates. Abator available. | Same | Smart design pulls all gas to the outside where it quickly breaks down and dissipates. Abator available. |
| Country of origin | Korea | Netherlands | Netherlands | Netherlands | USA | USA | USA |
| Price – 9/2022 | $11,800 | $47,081 | $60,958 | $64,708 | Very competitively priced. Contact us. | Same | Same |
| Not compatible with | Loads over 11 lbs (or 3.97 lbs for lumens), All H2O2 sterilizers: Items that can’t endure the higher temps required for plasma, liquids, powders, or strong absorbers (cellulose), delicate items that can’t withstand deep vacuum, dead-end or long lumens, ventilator tubing, implants. | Lumened instruments with <1 mm diameter and/or >10 m length (however PCD literature mentions 4 and 1.4 m…which should be more difficult to sterilize than the worst case load) See also the list of common H2O2 limitations to the left. | See to the left | See to the left | Food, drugs and liquids | Food, drugs and liquids | Food, drugs and liquids |
Two important notes:
On Sterlink: Keep in mind only the 36 min cycle is FDA-Cleared. They talk about sterilizing single-channel Teflon® / Polyethylene lumen >1.25mm and <600mm but that is NOT included on their FDA clearance.
As for the rest, it all depends on your needs. The functional capacity is very small (3.8x smaller). When calculating cost, keep in mind to calculate 3.8x the cost of all consumables – assuming you’ll be running at full capacity. So for instance their gas costs $5.50 a load x 3.8 =$20.90 in just sterilant (to sterilize the same volume).
On Tuttnauer: All of the PlazMax sterilizers are NOT FDA-Cleared and they don’t claim a sterility assurance level.
