
Medical Device
Sterilization made simple.
Andersen is proud to offer terminal sterilization solutions to many industries. All are competitively priced, easy to use and offer unparalleled versatility.
Tell Us Where you Work
Medical Device sterilization
game-changer
Medical devices are increasingly complex. Achieve terminal sterilization and unparalleled compatibility - even with mated surfaces, long narrow lumens or intricate designs. No matter where you are in the development process, we have a sterilization solution to fit your needs.
Commonly Sterilized Items
With Andersen, achieve 10-6 SAL terminal sterilization of:
- New devices undergoing prototyping, testing or trials
- Custom or semi-custom devices
- Devices with a short shelf life
- Polymer resin-based products
- Single-use medical devices
- Syringes
- Surgical staplers
- External sterilization of sealed pre-filled syringes, vials or cartridges
- Endoscopes, multi-channel devices with long narrow lumens
- Duodenoscopes
- Urological endoscopes
- Bronchoscopes
- Plastic or rubber devices
- Specula
- Stents
- Catheters
- IV Sets
- Plastic tubing & bloodlines
- Anesthesia masks
- Equipment with integrated electronics
- Heart valves & pacemakers
- Pumps
- Respirators
- Anesthesia circuits
- Surgical drills
- Fabric or cellulose items
- PPE
- Drape
- Wound care dressings
- Mixed-material items
- Procedure kits & surgical trays

Make Sterilization Part of Your Production Process

Consider purchasing an Andersen sterilizer for in-house sterilization if you:
- Require sterile processing of items in smaller lots
- Are performing research
- Have product under development, including clinical trials
- Have a product undergoing compatibility or functionality testing
- Manufacture custom devices and/or combination devices and drug products
Opting for In-House sterilization garners several benefits:
- Control every aspect of your sterilization workflow and improve your ability to manage inventory
- Reduced sterilization turnaround time
- Scalable, expand incrementally and virtually indefinitely based on your needs
- Reduced transportation costs and environmental impact
- Allowed in municipalities where other gas sterilizers are not, due to Andersen’s ultra-low emissions
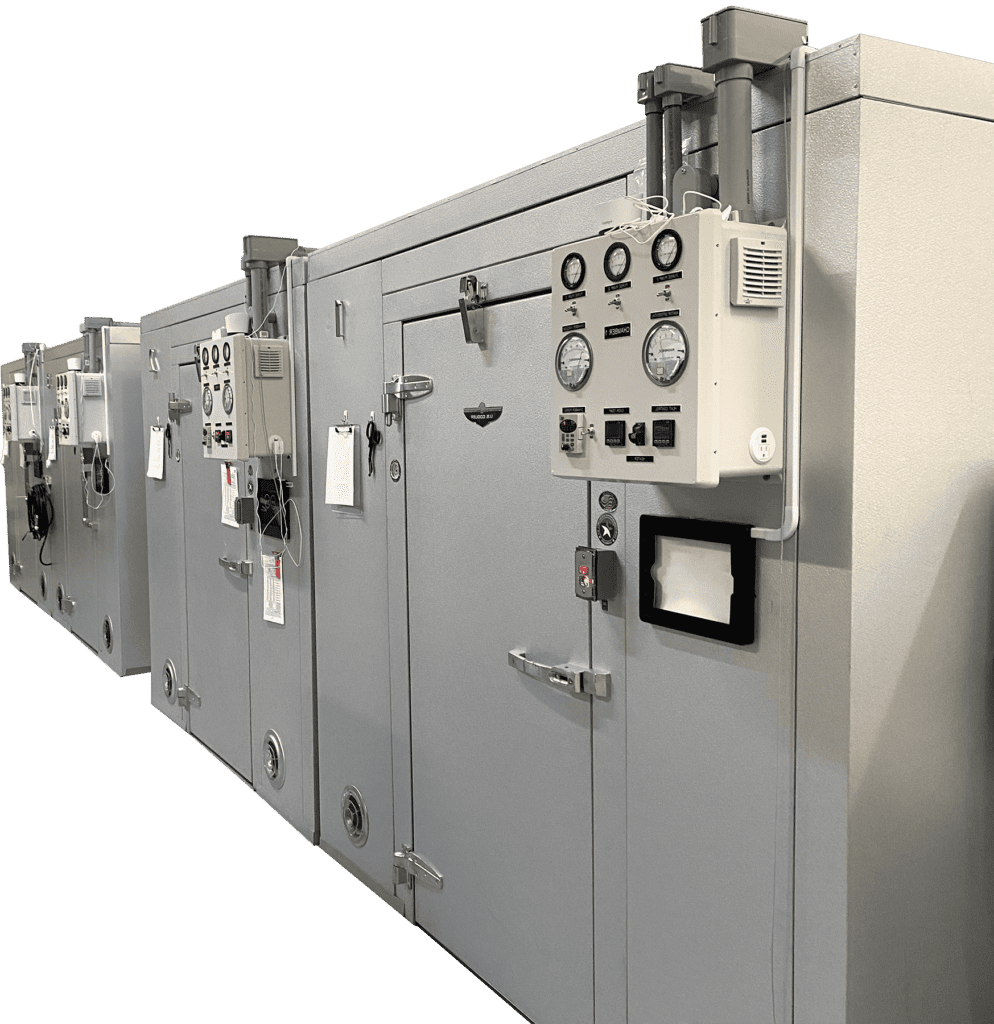
Three in-house sterilizer line choices

Anprolene
VALUE
A favorite of universities and research labs! This tabletop sterilizer is easy to install and operate. Validated to achieve a 10-6 SAL at room temp (<68ºF) with a 12- or 24-hour cycle.
State-of-the-Art Facility Offering Custom Cycles and Fast Turnaround
Consider contract sterilization at Andersen’s award-winning, FDA-registered and ISO-approved facility (Andersen Scientific) if you:
- Require sterile processing of devices in smaller lots (less than 2 pallets)
- Require new custom cycle development and/or validation
- Need sterilization testing of new devices
- Need package validations for sealers, pouches and other packaging systems
Opting for Andersen contract sterilization offers these benefits:
- In-depth R&D experience relative to sterilization
- Industry experts – Andersen representatives are longstanding members of the AAMI and ISO committees that create the standards used in the industry
- Rapid turnaround: devices/products can be sterilized and shipped back out within time frames, that meet your needs:
- Two weeks
- One week
- 24 to 48 hours
- Can customize cycles to meet your specific requirements
- Turnkey solution – finished product returned ready for distribution or can be shipped directly to customer/distributor
Services:
- Validation
- Sterilization process
- Packaging (including sealer)
- Shelf-life & transportation studies
- Residual testing
- Contract sterilization
- Lab services
TERMINAL STERILIZATION
Achieves FDA-required 10-6 sterility assurance level (SAL) for terminal sterilization of medical devices. In fact, we have 15 recent FDA clearances.
fda clearances
award-winning
Our exclusive EO-Flexible Chamber Technology and the resulting ability to use a microdose of EO has garnered two national awards.
Flexible
With Andersen you can choose in-house sterilization, contract or both. Any way you slice it, Andersen makes it so easy.
Right-sized
High capacity, small footprint. Sterilize a lot without taking up much space. From tabletop to refrigerator-sized - Andersen has a unit to fit your needs.
Scalable
Our in-house systems allow you to expand incrementally and virtually indefinitely based on your needs. You can also expand use of our contract option as needed.
Oversight
Control every aspect of your sterilization workflow and improve your ability to manage inventory.

In-house medical device case study
Challenges:
Over a decade ago, one of our manufacturing customers chose to bring sterilization in-house because they wanted control over every aspect of production and inventory management. They did not want to waste the energy and effort to ship product back and forth.
Solutions:
The company gained control over their supply chain and cut out shipping woes by purchasing an EOGas 333. By daily in-house sterilization of device production, small and medium manufacturers can achieve the aggregate capacity of one or more commercial EO pallet chambers.
Results:
With one cabinet the company reported they could sterilize up to 10 pallets of product per month. Our systems are inherently and uniquely scalable. As their demand grew they added additional EOGas 3 cabinets to achieve necessary capacity. Today, they run 10 EOGas 333 cabinets seven days a week. They can now sterilize up to 25 pallets per week or 100 pallets per month. Note: One way many customers gain efficiency is by only sterilizing the end product (not outer product/shipping packaging).
Contract medical device case study
Challenges:
When the COVID-19 pandemic hit, authorities began requesting testing supplies and Keystone Solutions Group knew they could help. From first request to sending out swabs – it was just four weeks.
Solutions:
“Andersen Scientific was willing to jump in and understood how to get there,” said David Furchak, Launch Architect at Keystone. Andersen expedited the validation and packaging of Keystone’s swabs starting in March 2020. “The employees at Andersen have been good about figuring out the best path forward. Their collaboration and willingness to have conference calls at all hours is the key to success.”
Results:
Andersen sterilized two pallets per week from Keystone for over four months – during the peek of the pandemic. Generally, we were able to returned the swabs the day after receiving them.


Why ethylene oxide (EO/EtO) sterilization?
Ethylene oxide is the gold standard in sterilization. It offers hospital-level sterility assurance (10⁻⁶) along with
unparalleled gentleness and material compatibility.


