IRIS = Bad Science
Ethylene oxide in the US
To understand the scale of ethylene oxide (EO or EtO) use, the total usage of EO as a sterilant in the US is about 14 million pounds per year. Commercial sterilization facilities generally have several chambers, use hundreds of pounds of EO per cycle and can operate 24 hours a day, 7 days a week.
Healthcare use is quite different. EPA estimates 0.1% of EO used for sterilization is used in small-sized facilities.
Our systems use at most 0.039 pounds (17.6 grams) per cycle. A typical user passes about 5 pounds of EO through the system in an entire year.
EO is Everywhere
“Ethylene oxide is a versatile compound that’s used to help make countless everyday products,” says the American Chemistry Council. “Ethylene oxide plays an important role in the development of batteries for electric vehicles and is used to support agriculture, oil and gas, as well as to develop semiconductors. Another important use of ethylene oxide is the sterilization of medical equipment. It is estimated that ethylene oxide sterilizes 20 billion medical devices each year, helping to prevent disease and infection.”
More uses of EO & its derivatives
- Personal care items (shampoo, laundry detergent)
- Durable fabrics – clothing, carpet, upholstery, pillows
- Carpet backing and furniture cushioning
- Medications/Pharmaceuticals
- Safety glass
- Automotive & architectural glass
- Roofing
- Appliance insulation
- Household cleaners
- Industrial cleaners
- Fungicides
- Antifreeze
- Brake fluid
- Lubricants
- Architectural coatings
- Automotive seating
- Natural gas and oil industry products
Sources of EO
Ethylene oxide (EO or EtO) is everywhere around you. It is naturally produced in your body and in the environment by plant decay.
Have you heard that you should not store bananas with apples? That’s because bananas off-gas enough ethylene to make apples ripen too quickly. So, yeah, that fruit bowl on your kitchen table? It is a source of ethylene gas, which is a precursor to EO.
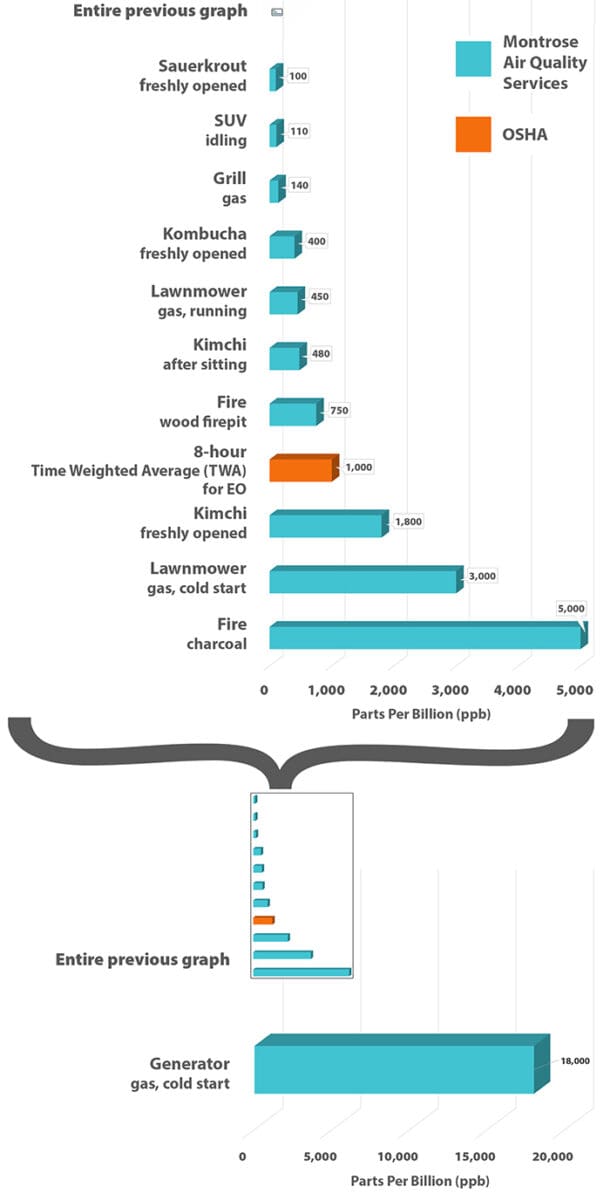
Speaking of food: Common fermented foods like Kombucha, Kimchi or Sauerkrout also emit well above the EPA’s “safe” level.
Internal combustion engines produce it too – your car and lawnmower as well as tractor trailers and planes.
If you smoke or light a campfire, those too are significant sources of EO.
It’s in the soil and in the air around us – produced entirely naturally or by processes we mostly take for granted in our modern world.
We don’t mean to trivialize exposure concerns. EO is toxic. It, and any other chemical used to kill all living microorganisms on medical devices, is not something people should be inhaling.
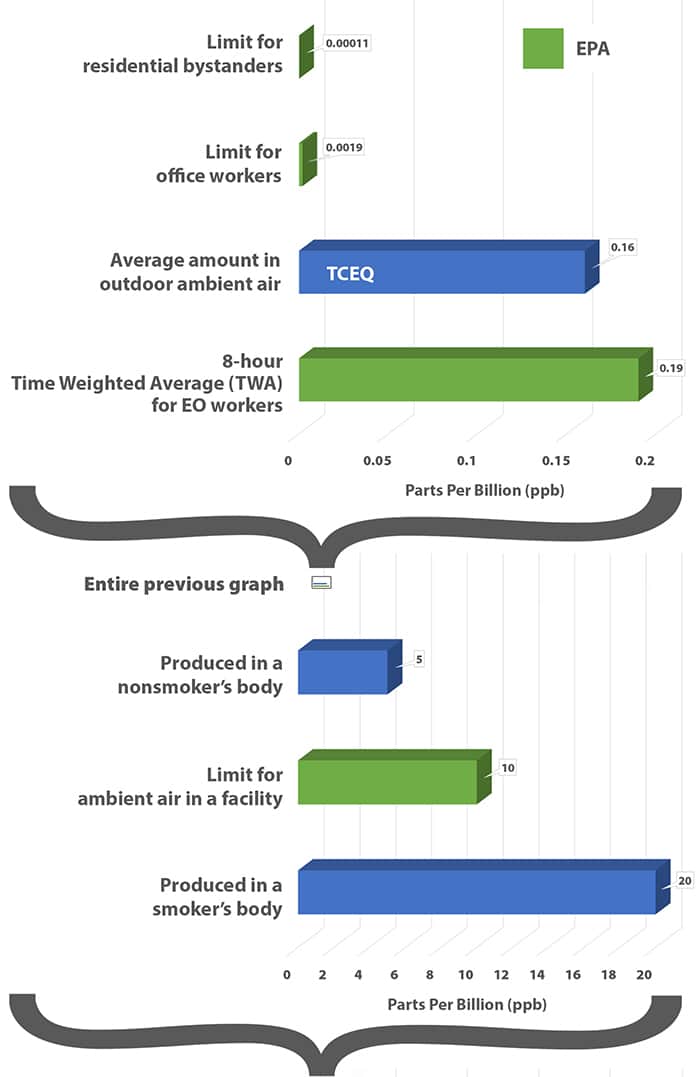
Occupational Safety and Health Administration – OSHA – the organization that monitors worker safety in the United States – has long set the EO exposure limit at 1 part per million (ppm) over 8 hours or 5 ppm over 15 minutes.
These new EPA proposals would set occupational limits at 10 parts per billion (ppb) and the “safe” residential bystander level would be set at 0.1 parts per trillion.
In order to compare apples to apples, in the graph to the right all units have been changed to parts per billion.
Side note: It is EPA’s job to monitor what is emitted from buildings, not to eat OSHA’s lunch by attempting to legislate worker exposure.
How did ePA calculate Risk?
Over the last few years, EPA’s risk assessment tool – Integrated Risk Information System (IRIS) – has become extremely controversial. To put it bluntly, is based on bad science and bad methodology. No less than the American Chemistry Council, National Academy of Sciences and more than a dozen members of Congress (1, 2) have called IRIS into question.
When EPA calculated the risk to bystanders and workers, they used existing data showing EO exposure levels for commercial sterilization workers between 1976 and 1985 and then extrapolated exposure rates of workers between 1938 and 1978, before EO was measured in the workplace. EPA assumed (extrapolated) the exposure rates for the earlier workers were the same as the later workers. After the study EPA used to calculate risk, researches followed the sterilization workers for 15-20 years to determine if there were delayed health effects from exposure.
For those earlier years, the EPA had to make assumptions about exposure because there was no data. They had to decide which statistical method they would use to extrapolate that data. This is where many dispute the validity of EPA’s IRIS model.
OSHA was founded in 1970 and set an EO exposure rate in 1971. Awareness of workplace safety and limiting exposure to harmful chemicals was a relatively new concept. So why would EPA assume that workers before the founding of OSHA and an exposure limit were subject to the same levels as those that came after?
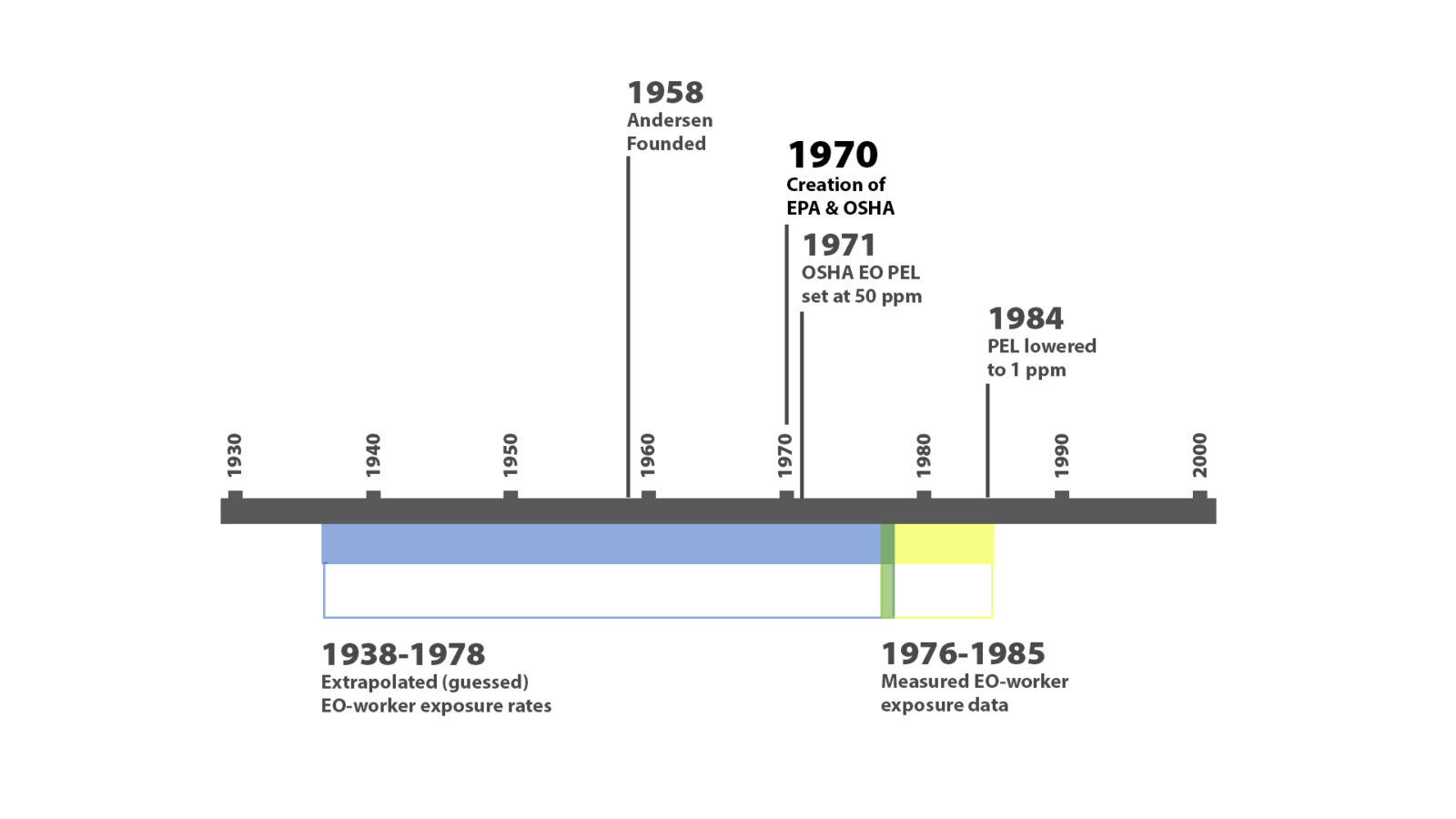
In the 1940s, for instance, we know the Army threw a tarp over a truck and simply released EO under the tarp. It was the “wild west” of EO-use in those days! It seems nearly certain that those earlier workers were exposed to far higher levels.
Regardless, the EPA set risk thresholds. What level of cancer risk is acceptable?
- For EO workers – acceptable cancer risk was set at 1 in 10,000
- For office workers – acceptable cancer risk was set at 1 in 100,000
- For residential bystanders – acceptable cancer risk was set at 1 in 100,000,000
However, when you do the math on that risk – given the assumptions they made above – IRIS spit out some comically low numbers.
you emit dangerous levels of EO
Here’s the thing about saying lifetime average EO concentration would need to be less than 0.11 parts per trillion for the safety of residential bystanders. There’s ample data showing how much EO is in the human body all the time. The body of a nonsmoker has about 5 ppb EO; smokers have more, about 20 ppb.
We cut out a high and a low outlier (the amount in a smoker’s body and the residential bystander limit) because they couldn’t be helpfully displayed on the same graph. Below we show the average amount in a nonsmoker’s body and the EPA’s proposed new limits.
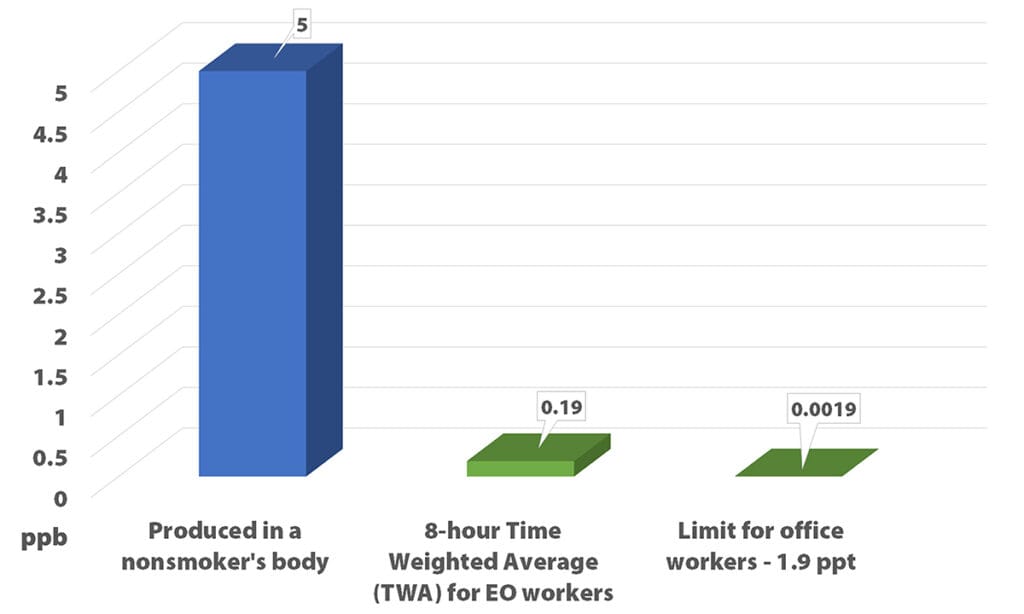
A recent letter signed by eight Congress members point out the proposed level is “19,000 times lower than naturally created levels of ethylene oxide in the human body and three to four orders of magnitude lower than levels from other sources measured in ambient air.”
Andersen Sterilizers’ President, William Andersen, MD, FAAOS, did a Pubmed search to see what the medical world had to say about EO toxicology. While he found plenty of articles calling IRIS into question, he found not one that corroborated its results.
At Andersen, we have a strong personal interest in the health of our customers. We join many experts who feel the IRIS EO risk assessment is deeply flawed, along with the proposed regulations based on it. Again, it is based on bad science and bad methodology.
Articles
September 28, 2023
Energy & Commerce Chair Rodgers
E&C Warns EPA: Don’t Make Medical Surgeries and Equipment Unsafe and Non-sterile
Three House committee and subcommittee chairs sent a letter to the White House outlining the risks for patients inherent in EPA’s recent proposals. “…we share FDA’s concerns that EPA’s proposed actions will cause significant disruptions in patient access to emergency care, including surgery instrumentation and pacemakers, and threaten patient safety with increasing hospital-borne infections.”
The letter also asked pointed questions about implementation, position and consequences. “Is it the Biden administration’s position that one medical risk in just a few communities should be prioritized over greater medical risks for all Americans?”


September 12, 2023
JDSUPRA
Lisa Baird
Delaware Is Definitive On No-Injury Medical Monitoring
Covers a State-level judgement, denying payouts for injuries that have not yet occurred.
“‘[The judgments] recognized that allowing ‘traditional, full-blown tort liability’ in the absence of an actual injury threatens ‘unlimited and unpredictable liability’ and a ‘flood’ of less important cases that could swamp the claims of those with injuries that do manifest, particularly because exposure to even toxic substances (fortunately) may never result in any harm.”
September 12, 2023
EHS Daily Advisor
Lisa Whitley Coleman
The Ongoing Battle Over EtO
“The EPA’s proposed rules on regulating ethylene oxide (EtO) have sparked a catfight between the EPA and the Food and Drug Administration (FDA), according to several sources.
‘We do have concerns’ about the EPA proposal, FDA commissioner Robert Califf testified to Congress in May,” Axios continues. “‘A sudden restriction would create substantial difficulty with critical medical devices. EPA is in the lead in this. We’re working on it. There’s an interagency process.'”


August 19, 2023
U.S. Senate Committee on Health, Education, Labor & Pensions
Ranking Member Cassidy Blasts Biden for Prioritizing Environmental Activism Over Americans’ Health
“[Dr.] Cassidy rebuked the Environmental Protection Agency (EPA) for its proposals to limit ethylene oxide (EtO), a chemical that is used to sterilize 50 percent of all medical devices and 95 percent of all surgical kits in the United States. EtO is the only sterilizer available for certain devices that cannot be sterilized with heat or radiation, such as endoscopes, syringes, and heart valves. These policies will have devastating impacts on domestic medical device manufacturing in the United States and exacerbate medical product shortages, threatening the lives of Americans who need access to these critical supplies. In pursuing these proposals, the EPA failed to address numerous concerns from the Food and Drug Administration (FDA) and the Department of Health and Human Services (HHS) on their impacts.”
Dr. Cassidy’s Letter to Present Biden & EPA Administrator Regan
July 27, 2023
Washington Legal Foundation: Legal Backgrounders
Robert E. Johnston, partner, & Aleksandra Rybicki, associate, at Hollingsworth, LLP – Washington, DC
ETHYLENE OXIDE: HOW DUBIOUS REGULATORY SCIENCE HAS FUELED VICIOUS CYCLE OF LITIGATION AND OVERREGULATION
“The proposed new requirements—which will be in addition to existing regulations—would be time-consuming, costly to implement, and would require substantial dislocation and downtime in a medical device sterilization industry that is already operating at full capacity to provide an indispensable service. The new proposed regulations also will give ammunition to plaintiffs’ lawyers to argue that these preventative measures should have been taken already, in many instances years before the issuance of the new regulations. Thus, EtO facilities that have historically been given a clean bill of health by their regulators will again be accused of causing cancer and potentially other ailments for not implementing such preventative measures sooner.”


July 13, 2023
AdvaMed
Gail Charnley, Ph.D.
Op-ed: Risk, Regulation, and Reality Checks: The Example of Ethylene Oxide
“Ethylene oxide is ubiquitous in the air we breathe; sterilization facilities in compliance with current laws contribute only a very small fraction of the ethylene oxide that people already breathe. Ethylene oxide comes from many natural and industrial sources. In fact, just one half of one percent of the ethylene oxide used in the U.S. is used for medical device sterilization. The rest comes from other commercial sources and from automobile exhaust, food preparation, consumer products, decaying plants—and from our own bodies, where we make it naturally and then exhale it. Today, by far, the largest source of ethylene oxide exposure is our own bodies, accounting for over ninety percent.”
July 12, 2023
MedTech Intelligence
AdvaMed Warns EPA of Massive Interruption to Patient Care
“In comments filed with the Environment Protection Agency (EPA), industry association AdvaMed called for continued cooperation between the medical device industry and the EPA as the regulations covering medical device sterilization using ethylene oxide (EtO) move forward. AdvaMed said if the proposals are finalized as written, the U.S. will see a massive interruption in patient care and access because of a 30% to 50% reduction in sterilization capacity for life-saving devices.”


July 10, 2023
American Chemistry Council
ACC Submits Comments on EPA’s HON Proposal
“We are particularly concerned with the EPA’s proposed requirements regarding ethylene oxide. Ethylene oxide is a versatile compound that’s used to help make countless everyday products. Ethylene oxide plays an important role in the development of batteries for electric vehicles and is used to support agriculture, oil and gas, as well as to develop semiconductors. Another important use of ethylene oxide is the sterilization of medical equipment. It is estimated that ethylene oxide sterilizes 20 billion medical devices each year, helping to prevent disease and infection.
We oppose any rulemaking that uses the EPA’s flawed IRIS value for Ethylene Oxide. ACC and others have detailed the severe science-based flaws with the IRIS value that resulted in an overly conservative value that is below background levels of ethylene oxide. In fact, the IRIS program’s proposed toxicity value is 19,000 times lower than naturally occurring levels of ethylene oxide found in the human body. EPA should await the outcome of litigation in the D.C. Circuit prior to proceeding to use the IRIS value in its risk assessment for EO.”