Andersen is Different
We believe imposing regulations on the healthcare providers, veterinarians, medical device manufacturers and research facilities that use our sterilizers is unnecessary because Andersen is DIFFERENT from any other sterilization company.
- Founded and led by medical doctors, with scientists on staff and boasting 18 FDA clearances since 2015 (with all the scientific rigor that is required) – Andersen uses proprietary technology demonstrated to be PRECISE, RELIABLE & PROVEN.
- This AWARD-WINNING technology recently received recognition from the FDA and others for our environmentally friendly and ultra-low emission sterilization process. We remain committed to protecting the environment.
- Andersen has a 60+ year history proving our systems are incredibly DEPENDABLE and EASY TO USE.
- We require operators to be trained on safety procedures before use and provide free USER TRAINING for the life of the sterilizer.
- USER SAFETY is one of Andersen’s prime tenants and the focus of much of our innovation over the years. Our sterilizers have many safeguards in place including our negative pressure cabinet which acts as a vent hood and our impermeable sterilization bag which serves as an incredibly gas-efficient chamber.
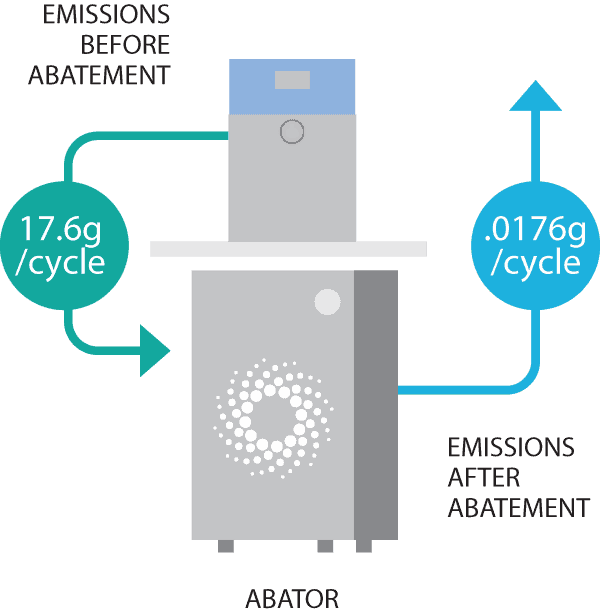
- Our sterilizers use a TINY AMOUNT of EO per cycle, 17.6 grams or less. This is 90% less than any other sterilizer on the market.
- Our customers are required to SAFELY VENT their sterilizer to the outside, 25 feet or more from air intakes and public walkways.
- When released into the atmosphere EO DISSAPATES QUICKLY. As the EPA says, “Ethylene oxide reacts in the air to form formic acid, which is a naturally occurring chemical.”
- Formic Acid is one of the simplest and most abundant organic molecules in our atmosphere.
- Unlike most other sterilization methods, we offer OPERATOR EXPOSURE MONITORS.
- There are no operator exposure monitors on the market (made by any company) capable of measuring parts per billion, which is the level at which they will need to monitor to meet the proposed EPA requirements.
- However, our monitors are very capable of measuring the levels OSHA and other regulatory bodies have currently placed on EO. We recommend you test when the sterilizer is installed, once a year, and anytime the sterilizer is moved.
- We offer an optional emissions abator that scrubs 99.9% of EO from the exhaust stream. With the abator, our sterilizers emit LESS THAN A QUARTER POUND OF EO A YEAR.
- Worst-case scenario, our largest (refrigerator-sized) sterilizer without an abator, running an un-realistic 24 hours/365 days a year, would produce 126.74 lbs. of EO. This is a minuscule amount of EO compared to the problematic contract sterilization facilities that have pumped tons of EO into the air each year…or, indeed, to any other sterilization system on the market.
Also keep in mind that the U.S. EPA, with the regulations currently on the books, does not require abatement of an EO emissions source until volume exceeds 2,000 lbs.
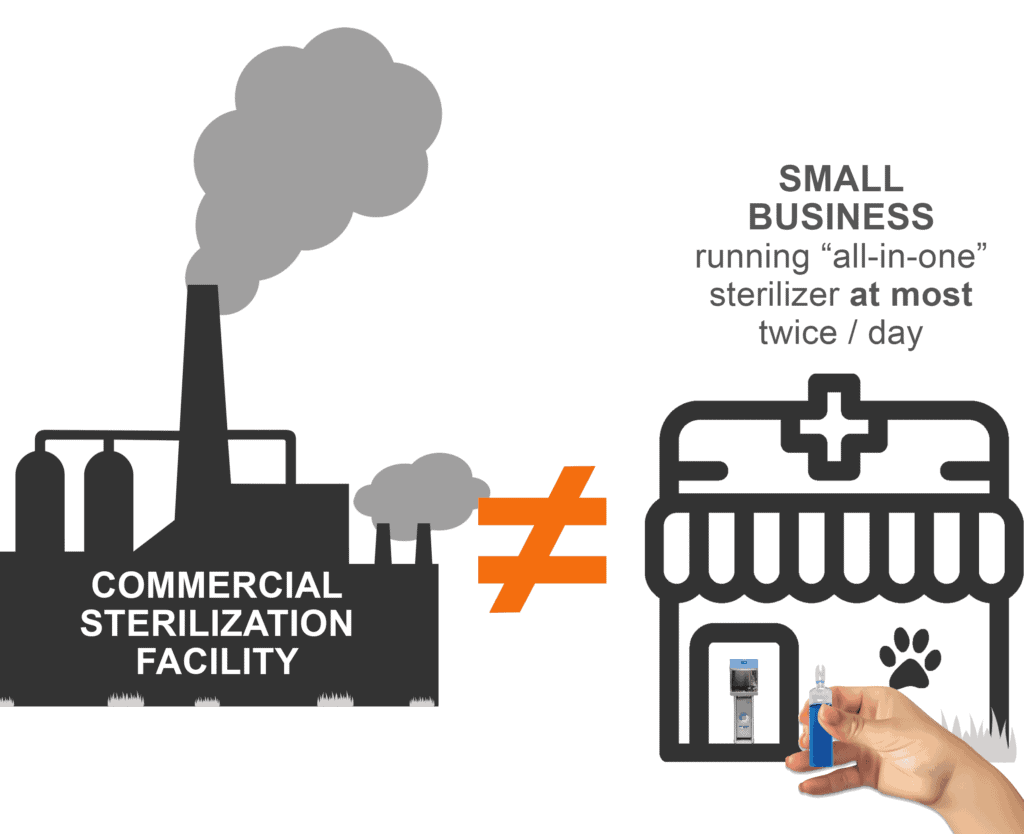
It is absurd to apply the same regulations to workers who spend their entire working life in an incredibly EO-dense environment and to employees of small businesses who empty one of our highly efficient and smartly designed machines at most twice a day. For contract sterilization facilities, sterilization is their business. It happens all day, every day. To our customers, sterilization is a small, yet critical, part of their business.
So, bottom line: These regulations which were written with large commercial sterilization facilities in mind could potentially have a significant impact on your business. We are working hard to mitigate any impact.
We made all the points outlined above and more in our comments to the EPA, during the official public comment period which ended June 12. We also joined the efforts of EOSA, AdvaMed and three other industry organizations in encouraging the EPA to consider the extensive data on EO, the decades of its safe use and its role as a substance vital to public health.
Draft regulations like this often undergo a great deal of change before being put in place, and occasionally never go on the books at all. We feel that there is a good chance that the final version of these regulations will not apply to Andersen’s customers.
Regardless, we will keep you updated via posts on these pages and social media.
NEXT STEPS
iris = bad science
The EPA’s risk assessment tool – IRIS – is deeply flawed & controversial