Comment
Thank you for letting your voice be heard! After all, that’s exactly what the comment period is for.
Hearing from you will make such a difference. The EPA needs to know that their proposed regulations would impact businesses like yours, not just commercial sterilization facilities.
Below is a draft letter you can update to reflect your experience and opinion. If you would please consider submitting your updated letter to the EPA, we would be deeply grateful. As a reminder, the deadline for comments is June 27.
NOTE: We intend to email out an individualized draft comment to the EPA to all our current customers. If yours does not make it to you, please reach out to your Account Manager. We can give you the amount of EO you emitted last year.
Please feel free to change it and make it your own. The EPA says, “A constructive, information-rich comment that clearly communicates and supports its claims is more likely to have an impact on regulatory decision making.” They also encourage people to talk about impacts to business.
Specifically, EPA is seeking public comment on:
- “…the feasibility of respirator use (supplied airline respirators or self-contained breathing apparatus respirators) in
healthcare facilities for employees who are unloading EtO sterilization equipment from the sterilization chamber.” (p. 76)- Keep in mind that “healthcare” includes veterinary practices and single practitioners like dentists and plastic surgeons.
- The EPA has asked if there a way that you could lay out your facility to make a Supplied Airline Respirator (with its
hose attaching a person to a wall) feasible or if you could manage employee shifts so the 45-minute air canisters of a
Self-Contained Breathing Apparatus would not be burdensome (p.74)? - They mention staff leaving the building as a third option (p. 72).
- What about purchasing and maintaining an instrument capable of measuring parts per billion, would that impact your
business (p. 74)?
Again, the below is just a starting point. Bolded information must be replaced with your own.
EPA,
My name is Sarah Smith and I am the Practice Manager at ABC Veterinary based in San Francisco.
I am reaching out in regard to your proposed ethylene oxide regulations currently in their comment period (EPA-HQ-OAR-2019-0178).
We are a customer of Haw River, N.C.-based Andersen Sterilizers. We own one of their highly efficient “all-in-one” tabletop sterilizers and it is a vital part of our infection control process.
As a small business that emitted just a few lbs of EO last year, for such an important reason (protecting our patients and preserving our instruments), we are concerned that we will be unfairly regulated side-by-side with large commercial sterilization facilities under the proposed regulations.
The regulations as currently written would create a significant cost burden to our business: the changes to our building, monitoring exposure to 10 ppb (if required) and the elaborate PPE. Any one of these would have an enormous impact on us economically, on our ability to continue to provide superior patient care and in our fight against both common infection and multi-antibiotic resistant bacteria.
We join Andersen Sterilizers, the Ethylene Oxide Sterilization Association (EOSA), the Advanced Medical Technology Association (AdvaMed) in encouraging you to consider all the available data and input from companies such as ours as you make vital decisions to protect public health and worker safety while not disrupting medical device supply or negatively impacting patient care and safety.
Please consider retaining the current health care exemption. Otherwise, there should be a cut-off level that recognizes extremely low volume users like us.
Sincerely,
Sarah Smith
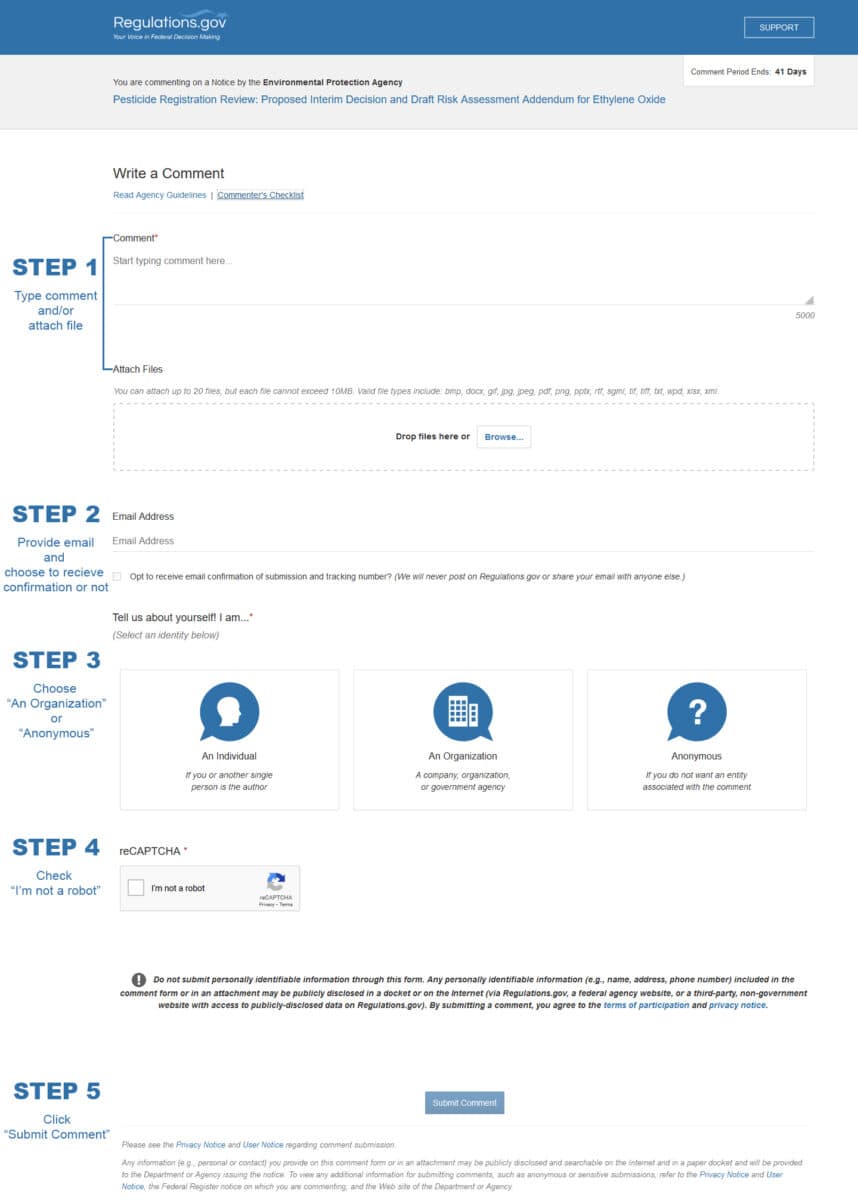
Click the button above visit the EPA comment page. Below is a quick how-to using a screen-grab of the page. When you have your comment ready, submitting it will only take about two minutes.
In Step 1 you can simply copy your pre-written comment and paste it into the comment field and/or attach it.
Note: Near the top of the submission page shown below, you will find some guidelines (rules) and a checklist (hints for a good submission). If you have problems with the page, please reach out to their help desk at 1.866.498.2945 or regulationshelpdesk@gsa.gov
NEXT STEPS

we’re DIFFERENT
Andersen is different from every other sterilization company, in many key ways



