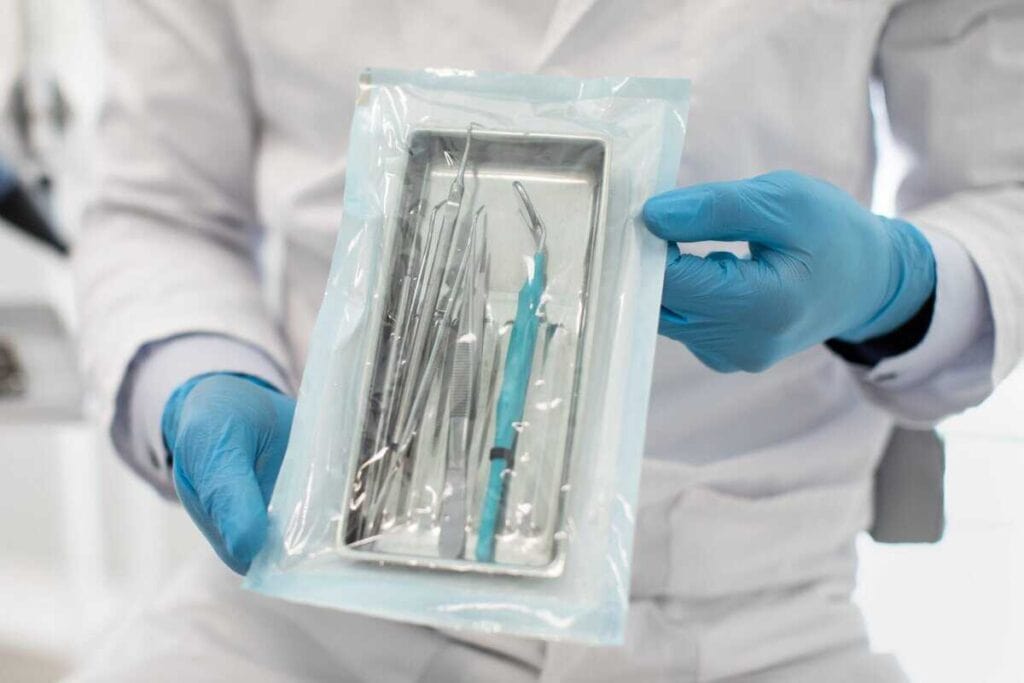
In the high-stakes environment of medical device manufacturing and healthcare facilities, achieving a 10⁻⁶ Sterility Assurance Level (SAL) is not merely a regulatory hurdle but a fundamental commitment to patient safety. The terminal sterilization process represents the ultimate safeguard, ensuring that products are rendered free of viable microorganisms within their final, sealed packaging to eliminate the risk of post-process contamination. At Andersen Sterilizers, we’ve dedicated over 60 years to perfecting the tools and techniques that protect patients, staff, and entire facilities through validated terminal sterilization methods.
Modern healthcare requires a sophisticated balance between aggressive microbial elimination and the delicate preservation of heat-sensitive instruments. Our proprietary EO-Flexible Chamber Technology optimizes this balance by utilizing advanced gas diffusion to provide deep penetration while significantly reducing gas consumption and emissions.
Key Takeaways
Terminal sterilization within final packaging is the gold standard for achieving a 10⁻⁶ Sterility Assurance Level (SAL), eliminating post-process contamination risks while meeting stringent FDA requirements.
Ethylene Oxide (EO) provides a critical balance of high microbial lethality and material compatibility, making it the industry standard for sterilizing heat-sensitive electronics and complex polymers without causing degradation.
Our proprietary EO-Flexible Chamber Technology reduces gas consumption by up to 90 percent and lowers environmental emissions by utilizing a localized vacuum that collapses around the load.
Bringing advanced EO sterilization in-house optimizes operational efficiency by reducing turnaround times, lowering logistical expenses, and extending the lifespan of high-value medical assets.
Terminal Sterilization Process Definition
The terminal sterilization process refers to sterilizing a product after it has been packaged and sealed in its final container. Unlike aseptic processing, where sterilized components are assembled in a sterile environment, terminal sterilization sterilizes the entire package, ensuring that both the contents and their packaging remain free from contamination throughout storage, transport, and until the moment of use.
This method is used for healthcare applications, including medical devices, surgical instruments, syringes, implants, and certain pharmaceuticals. It serves as the final barrier that ensures the product delivered to the patient is completely sterile with a validated 10⁻⁶ SAL, representing a one-in-a-million chance of a single viable microorganism remaining on a product after sterilization.
Critical characteristics of terminal sterilization:
Final packaging sterilization—Products are sterilized in their sealed containers, eliminating post-process contamination risks.
10⁻⁶ Sterility Assurance Level – Meets the most stringent FDA requirements for validated sterility
Complete barrier protection – Maintains sterility throughout the entire supply chain
Superior safety profile—Provides higher assurance than aseptic processing methods
Why the Terminal Sterilization Process Matters
Patient Safety and Microbial Lethality
When products are introduced into the body, such as catheters, surgical tools, or IV tubing, even the smallest microbial contamination can have devastating consequences. The terminal sterilization process eliminates this risk by processing medical devices in sealed packaging, avoiding any chance of recontamination during handling, storage, or transport.
Our EO-Flexible Chamber Technology acts as a powerful alkylating agent, disrupting the DNA of microorganisms while remaining gentle on the base materials of sophisticated equipment. By prioritizing this methodology, facilities leverage a superior safety profile that simplifies supply chain logistics while achieving total microbial lethality.
Regulatory Compliance and FDA Requirements
Organizations such as the FDA, CDC, and international regulatory bodies require stringent standards for sterility validation. The terminal sterilization process provides a validated and repeatable method of ensuring products meet or exceed these standards. Our EO sterilizers are FDA-cleared and ISO-certified, aligning perfectly with strict regulatory requirements for achieving and maintaining a 10⁻⁶ SAL.
Terminal sterilization offers a higher Sterility Assurance Level than aseptic processing, with a lower chance of human error or contamination during packaging. This is why regulatory bodies favor terminal sterilization over aseptic processing methods wherever feasible.
Operational Efficiency and Cost Management
Sterilizing products in their final packaging reduces the need for additional sterile handling environments. Bringing advanced EO sterilization in-house optimizes operational efficiency by reducing turnaround times and lowering logistical expenses associated with off-site contract sterilization services. Facilities can achieve rapid turnaround times for high-value assets while maintaining total control over cycle parameters.
Achieving Sterility Assurance Through Ethylene Oxide
Ethylene Oxide (EO) remains the industry standard for the terminal sterilization process because of its unmatched ability to permeate complex device geometries without the damaging effects of high heat or moisture. When managing delicate instruments such as endoscopes or multi-lumen catheters, facilities must ensure a Sterility Assurance Level of 10⁻⁶ without compromising the physical integrity of sensitive polymers or electronics.
This vapor phase process disrupts the DNA of microorganisms while remaining gentle on the base materials of sophisticated equipment. By choosing this method, facilities effectively eliminate the risk of post-packaging contamination while extending the lifespan of high-value inventory.
Advantages of EO for terminal sterilization:
Superior material compatibility – Protects heat-sensitive polymers, electronics, and delicate optics from thermal damage
Deep penetration capability—Gas reaches complex lumens, mated surfaces, and intricate internal geometries
Low-temperature processing—Operates at ambient temperatures to prevent warping, melting, or material degradation
Validated efficacy—Achieves 10⁻⁶ SAL with FDA clearance and proven scientific reliability
Extended device lifecycle—Prevents thermal stress and oxidative damage, reducing replacement costs
To understand more about key methods of terminal sterilization and how they compare, consider how EO’s unique properties make it the preferred choice for complex medical devices.
Proprietary EO-Flexible Chamber Technology Advantages
Our proprietary EO-Flexible Chamber Technology redefines the efficiency of the terminal sterilization process by fundamentally altering how gas is utilized. Unlike traditional rigid chambers that require massive volumes of gas to fill empty voids, our system uses a flexible liner that collapses around the load.
This targeted approach significantly reduces gas consumption, often by as much as 90 percent compared to conventional methods. Facilities can achieve a validated 10⁻⁶ Sterility Assurance Level while maintaining a much smaller chemical footprint.
Key advantages of EO-Flexible Chamber Technology:
90% gas reduction—Uses a fraction of the sterilant required by traditional bulk systems
Ultra-low emissions—Drastically lowers environmental impact and simplifies regulatory compliance
Localized vacuum design – Creates targeted penetration into recessed areas and long channels
Reduced operational overhead – Often falls below thresholds requiring complex abatement equipment
Enhanced staff safety – Minimizes technician exposure through controlled, contained gas delivery
FDA-cleared reliability—Maintains full regulatory compliance while optimizing efficiency
By utilizing significantly less gas per cycle, facilities can drastically lower emissions and simplify their regulatory compliance profile. This lean approach to sterilization does not sacrifice safety, as the process remains fully FDA-cleared and scientifically robust.
Learn more about our low-temperature, low-dose, low-emissions approach to terminal sterilization and how it transforms facility operations.
Balancing Material Compatibility With Lethality Requirements
Achieving a Sterility Assurance Level of 10⁻⁶ requires a rigorous terminal sterilization process that must not compromise the structural integrity of modern medical devices. Facilities must manage the narrow margin between ensuring total microbial inactivation and protecting sensitive polymers from oxidative stress or thermal degradation.
Critical materials requiring specialized terminal sterilization:
Heat-sensitive polymers – Plastics and composites that warp or degrade under autoclave temperatures
Electronic components – Pacemakers, diagnostic tools, and powered surgical instruments
Fiber optics – Endoscopes and visualization systems with delicate optical elements
Drug-coated devices – Stents and implants with therapeutic coatings vulnerable to heat
Multi-lumen catheters—Complex internal geometries requiring deep gas penetration
Pre-assembled surgical kits – Combination devices with multiple material types
Our EO-Flexible Chamber Technology offers a sophisticated solution by optimizing gas concentration and humidity within a localized environment. This targeted approach allows facilities to achieve complete lethality without exposing delicate components to the excessive heat or high pressure associated with steam or radiation.
Because the flexible chamber adapts to the specific load, it minimizes the volume of gas required while ensuring deep penetration into complex lumens. This method ensures that advanced electronics remain functional and reliable after every sterilization cycle.
Terminal Sterilization Process vs. Aseptic Processing
While both the terminal sterilization process and aseptic processing aim to ensure sterility, they differ significantly in their approach and reliability.
Terminal sterilization:
Sterilizes the medical device after it is sealed in final packaging
Achieves higher Sterility Assurance Level (SAL of 10⁻⁶)
Eliminates post-process contamination risks during handling and storage
Lower human error potential
Aseptic processing:
Assembles sterilized components in a sterile environment
Does not sterilize the final container-product system
Requires extensive environmental controls and monitoring
Higher risk of contamination during assembly
The terminal sterilization process offers a higher Sterility Assurance Level and eliminates the variables associated with human handling during aseptic assembly.
Applications of the Terminal Sterilization Process
Medical Devices and Surgical Instruments
Single-use medical instruments like catheters, scalpels, and tubing sets are typically terminally sterilized to guarantee sterility during surgical procedures. Our EO sterilization systems accommodate a wide range of surgical instruments, ensuring complete sterility without material degradation.
Syringes and Injectable Products
EO sterilization through the terminal sterilization process is frequently used for pre-filled syringes and needle-based systems, where heat and moisture could damage the components or compromise drug stability.
Implants and Prosthetics
Orthopedic implants, pacemakers, cardiac stents, and other internal devices must be completely sterile at the time of implantation. The terminal sterilization process ensures sterility until the moment of use, eliminating any risk of introducing infection into the patient’s body.
Laboratory and Diagnostic Equipment
Diagnostic kits, sample containers, and laboratory consumables must remain uncontaminated throughout storage and use. With the terminal sterilization process, these items are ready to use right out of the package.
Why Choose Andersen Sterilizers for Terminal Sterilization
At Andersen Sterilizers, we’ve been leaders in sterilization innovation for over 60 years. Whether you’re a hospital, a medical device manufacturer, or a research lab, we provide scalable EO sterilization systems that support terminal sterilization needs with unmatched efficiency and reliability.
What sets our terminal sterilization systems apart:
Low-temperature EO systems – Ideal for heat-sensitive and moisture-sensitive devices
EO-Flexible Chamber Technology – 90% reduction in gas consumption and emissions
Compact designs—Fit easily into existing facilities without extensive infrastructure modifications
FDA-cleared reliability – Full regulatory compliance with proven scientific validation
Advanced safety features – Protects staff and surroundings with engineered containment systems
Our goal is to help save lives by ensuring the highest standards of infection control through validated terminal sterilization processes.
Partner With Us for Terminal Sterilization Excellence
Integrating FDA-cleared terminal sterilization into your facility represents a decisive commitment to achieving a 10⁻⁶ Sterility Assurance Level while protecting your most valuable assets. Our proprietary EO-Flexible Chamber Technology effectively eliminates microbial life without subjecting sensitive electronics or delicate optics to the destructive heat and moisture of traditional autoclaving.
Whether you’re a healthcare provider, laboratory, or medical device manufacturer looking to implement or upgrade terminal sterilization systems, we offer customized sterilization solutions and expert guidance every step of the way.
Contact us today to speak with one of our sterilization specialists about how we can optimize your terminal sterilization process and help you achieve superior patient safety outcomes.
Frequently Asked Questions
What does a 10⁻⁶ Sterility Assurance Level (SAL) mean for medical device manufacturing?
Achieving a 10⁻⁶ SAL signifies a one-in-a-million chance of a single viable microorganism remaining on a product after the terminal sterilization process. This industry standard ensures facilities meet the most stringent FDA requirements for patient safety and validated sterility.
Why is the terminal sterilization process preferred over other microbial control methods?
The terminal sterilization process occurs within the final, sealed packaging, which eliminates the risk of post-process contamination during handling or storage. This methodology simplifies supply chain logistics while providing a superior safety profile.
How does EO sterilization protect heat-sensitive instruments?
EO acts as a powerful alkylating agent that disrupts microbial DNA at low temperatures, making it ideal for delicate polymers and electronics. This vapor phase process allows facilities to achieve total lethality without the material degradation associated with high heat or moisture.
What advantages does the proprietary EO-Flexible Chamber Technology offer?
Our technology utilizes advanced gas diffusion to optimize the balance between microbial lethality and instrument preservation. Facilities benefit from deep penetration into complex geometries while significantly reducing gas consumption by up to 90 percent.
Can EO effectively sterilize devices with complex internal geometries?
Yes, EO is the industry standard for its unmatched ability to permeate multi-lumen catheters and sophisticated endoscopes. The terminal sterilization process using EO ensures complete sterilization of intricate internal surfaces that other methods cannot reach effectively.
How does this sterilization approach impact operational cost-efficiency?
By utilizing EO-Flexible Chamber Technology in the terminal sterilization process, facilities reduce the volume of sterilant required per cycle, leading to lower overhead and improved resource management. This scientifically reliable solution protects high-value assets and extends the lifespan of sophisticated equipment.
Spread the Word
Expert Guidance for Your Needs
Tell us about your sterilization requirements — our experts are here to guide you to the perfect EO solution.