Every month, Andersen’s Ted May will explore topics like safety, history, emissions and even molecular science related to medical device gas sterilization.
These articles are published as part of Beyond Clean’s “Expert Series”—articles written by medical experts tackling some of the most pressing issues facing Sterile Processing departments. Beyond Clean is the global voice for sterile processing, connecting individuals through innovative and disruptive platforms such as podcasting, social media and new technology.
Scroll down to browse through all posts or skip to: Did Superbugs Disappear? Ι First Duodenoscopes, then Urological Endoscopes…Now Bronchoscopes? Ι Do you Know When to Use a PCD or Test Pack? Ι Do You Know Your Chemical Sterilants? Ι Is Disinfection Good Enough Ι Hospital EO and the Automobile
Is Liquid Chemical Sterilization Efficacy the Same as Ethylene Oxide Sterilization?
Ethylene oxide (EO) and liquid chemical sterilization (LCS) are typically used for devices that would be sensitive to high heat used in steam sterilization, as well as rubber and plastic devices that can be damaged by irradiation.
Does that mean EO and LCS efficacy are one in the same?
LCS: FDA describes LCS as a 2-part process: 1) Immerse the device in a liquid chemical germicide. 2) Terminally rinse the device with water to remove chemical residues.1 Displayed in Table 1, LCS has several limitations.
EO sterilization: In contrast, EO achieves “traditional sterilization,” which FDA defines as a “validated process used to render a product free of all forms of viable microorganisms.” According to FDA, for many medical devices, “sterilization with (EO) may be the only method that effectively sterilizes and does not damage the device during the sterilization process.”


LCS sterility assurance level: FDA states, although “the rinse water is treated to minimize any bioburden, it is not sterile” and therefore devices rinsed with this water cannot be assured to be sterile. “Furthermore, devices cannot be wrapped or adequately contained during processing in a liquid chemical sterilant,”1 rendering the devices prone to re-contamination.
EO Sterility assurance level: EO gas sterilization processes are designed by manufacturers with an associated sterility assurance level (SAL) of 10-6 — a level, according to the CDC, liquid chemical sterilants may not convey.2
Biological Indicator use with LCS vs. EO: Another salient limitation, FDA states that biological indicators (BIs) are “not appropriate” for monitoring a LCS process (Table 1).1 FDA notes further that BIs “are generally used for monitoring traditional sterilization processes, like EO sterilization, where a SAL 10-6 is achieved. FDA has not cleared any (Bis) for monitoring (LCS) process.”1
The survival kinetics for low temperature sterilization methods (gas, vapor or plasma) and for thermal sterilization methods (steam and dry heat) have been studied and well characterized. Federal agencies acknowledge, the kinetics for sterilization with liquid sterilants are less well understood. According to the CDC, “the survival curves for liquid chemical sterilants may not exhibit log-linear kinetics and the shape of the survivor curve may vary depending of the formulation, chemical nature and stability of the liquid chemical sterilant.”2
A comparative study: Rutala et al. (1998) compared the sporicidal activity of four different low-temperature instrument processing technologies including two using EO with hydrochloro-fluorocarbons and a liquid chemical sterilant, respectively.3 These researchers reported that the former was “highly effective” in killing approximately 106 resistant Bacillus stearothermophilus spores present in the center of narrow-lumen stainless steel tubes.” This study found, however, that the liquid chemical sterilant process “was not effective in completely eliminating the 106 inoculum under test conditions.”
Unlike LCS, EO sterilization is a traditional sterilization technology that is associated with a SAL of 10-6, can be routinely monitored biologically, yields a dry, wrapped processed instrument that can be stored sterile with a shelf life.
Did Superbugs Disappear?
“Superbugs” dominated industry headlines from 2015 onward. But over the past year and a half, COVID-19 has overshadowed the infectious disease news. Did superbugs disappear?
Superbugs are strains of bacteria, viruses, parasites and fungi that are resistant to most antibiotics and other medications commonly used to treat the infections they cause. These classes of antibiotics can include carbapenems – which are “last resort” antibiotics used to treat many types of serious infections caused by multidrug-resistant bacteria.1
Carbapenem-resistant Enterobacteriaceae – or CRE – are a particularly pernicious family of gram-negative bacteria resistant to these antibiotics. CRE and related superbugs have become a global public health scourge. The mortality rates for patients infected with CRE and certain other superbugs, particularly bloodstream infections, can be as high as 50% – or higher, in some patient subgroups.2,1
In February 2015, FDA acknowledged for the first time that reprocessed duodenoscopes could remain persistently contaminated with life-threatening superbugs.3 Earlier this year, FDA acknowledged other types of flexible endoscopes, particularly bronchoscopes and urological endoscopes, may pose a risk of infecting patients with superbugs and related multidrug-resistant organisms (MDROs).4,5 Indeed, according to the CDC, more healthcare-associated outbreaks have been linked to flexible endoscopes than to any other type of medical device.6
Ratcheting up the stakes, a report published in 2019 linked a duodenoscope to the possible transmission of a superbug carrying the mcr-1 gene, which can confer colistin antibiotic-resistance to the microorganism.7 Like carbapenems, colistin may be used as a “last line of defense” for treating some types of multidrug-resistant infections. Notably, some colistin-resistant infections may be untreatable.
The FDA has repeatedly (most recently in June) recommended ethylene oxide (EO) gas sterilization over high-level disinfection for reprocessing these endoscopes because of its “greater safety margin.”5 Numerous studies have documented that EO sterilization of endoscopes during infectious outbreaks has been associated with terminating these outbreaks. 8
Superbug infections associated with reprocessed endoscopes have certainly not disappeared. They remain a concern, warranting attention and enhanced measures to mitigate risk. Implementation of EO sterilization is an effective tool for terminating endoscope-associated CRE and MDRO outbreaks. A modern EO gas sterilizer has earned its place in a well-equipped SPD.

Superbugs
First Duodenoscopes, then Urological Endoscopes… Now Bronchoscopes?
Did you think endoscope reprocessing woes ended with the duodenoscope crisis of 2015? FDA doesn’t think so.
In June 2021, FDA published new guidance recommending sterilization of bronchoscopes over high-level disinfection (HLD) ― making them the most recent in a string of complex devices that cannot be dependably disinfected and have caused a rash of deadly superbug infections.
In February 2015, the FDA acknowledged duodenoscopes could remain contaminated with life-threatening superbugs after HLD reprocessing due to their complex design. It ominously stressed, “meticulously cleaning duodenoscopes prior to high-level disinfection should reduce the risk of transmitting infection but may not entirely eliminate it.”1 FDA found many of the superbug infections occurred despite cleaning and disinfecting the duodenoscope in accordance with manufacturer IFUs.
A few months later, FDA advised facilities to implement one or more of four supplemental measures, which included ethylene oxide (EO/EtO) gas sterilization. It said, “When possible and practical, duodenoscopes should be sterilized due to the greater margin of safety provided by sterilization.”2 Subsequent field surveillance and validation studies suggested that EO gas sterilization was the most effective of the recommended supplemental measures.3
FDA published alerts in 2019 and 2020 recommending that healthcare facilities and manufacturers “begin transitioning to duodenoscopes with disposable components” and, in the later alert, stressed “sterilization, particularly terminal (e.g., gas) sterilization, provides a greater margin of safety than high level disinfection.” It didn’t end there.
In April 2021, FDA issued a letter about the risk of infection associated with reprocessed urological endoscopes after receiving over 450 reports of patient infections and three reported deaths (outside of the United States). FDA concluded both IFUs and urological endoscope designs could be factors. Similar to earlier alerts, the FDA advised facilities to follow IFUs, maintain their devices correctly and gave two reprocessing choices – one of which included sterilization.5
Reprocessed bronchoscopes have now joined the growing family of complex devices linked to serious infections. The June FDA alert on bronchoscopes recommends healthcare facilities “consider using sterilization instead of high-level disinfection when feasible, because sterilization has a greater safety margin than high-level disinfection.”6
The threat of multidrug-resistant bacteria, including CRE, is becoming ever more serious. The evidence is mounting that HLD is not “good enough” for reprocessing many types of endoscopes. For the modern SPD, EO gas sterilization has returned as an essential tool in the fight to maintain patient safety.
4.

5.

6.

Do You Know When to Use a PCD or Test Pack?
Do you know the difference between a biological indicator (BI) and a test pack (also called a process challenge device or PCD) and the proper use of each in a sterile processing program?
Biological indicator: A successful sterilization process is verified with a biological indicator. BIs contain active bacterial spores, which provide a defined resistance to a sterilization process. The complete destruction of all the BI’s spores, often over one million of them, provides assurance that sterilization was achieved. Medical equipment that has been properly cleaned is typically never contaminated with organisms more resistant to sterilization and harder to destroy than a BI’s spores.
BIs demonstrate the microbial lethality of the process, providing a degree of confidence that sterility, as defined by the process’ sterility assurance level (SAL), was achieved. A sterilizer’s validated SAL of 10-6, for instance, describes the probability of only one resistant bacterial spore, from an original population of one million, surviving the process.

The problem is, a BI’s “negative” result does not, by itself, confirm that the processed item, particularly a complex device, is sterile. Why? A negative BI result only indicates that all resistant spores were destroyed at the site where the BI was placed.
When processing a complex instrument, the challenge is to confirm that every microorganism deep within the load itself – for example, within the long and narrow internal channels of a flexible endoscope – was destroyed. How do you place a standard BI in the middle of an endoscope’s longest and narrowest channel?
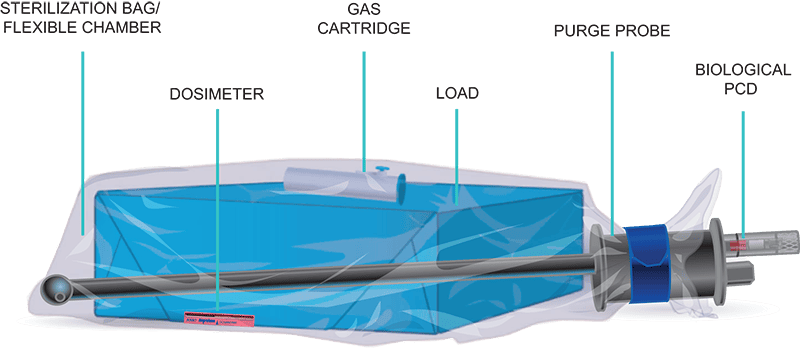
Process challenge device: This confirmation is resolved using a unique accessory called a process challenge device, or PCD. Also referred to as a “BI test pack,” the PCD is validated to be more difficult to sterilize than the “worst-case” location of a given load.
Moreover, the PCD standardizes practice by eliminating the requirement that staff consistently place the BI at the same, proper site within the sterilizer prior to each cycle, a technique that can vary and cause misinterpretation of the BI’s result.
How does your practice utilize PCDs or test packs? Do you have a PCD that is validated for especially complex devices such as duodenoscopes or are you assuming that a BI placed in the load is “good enough?”
Deeper Dive: Want to know more?
At Andersen, we don’t ask customers to just trust their cycle was successful. We have a long and proud history of offering cycle monitoring – and peace of mind. We offer biological indicators for use with all of our systems and several of our systems also come standard with an integrated Process Challenge Device – Anprolene AN75 and both our EOGas 4 and EOGas 4 – 6 hour sterilizers.
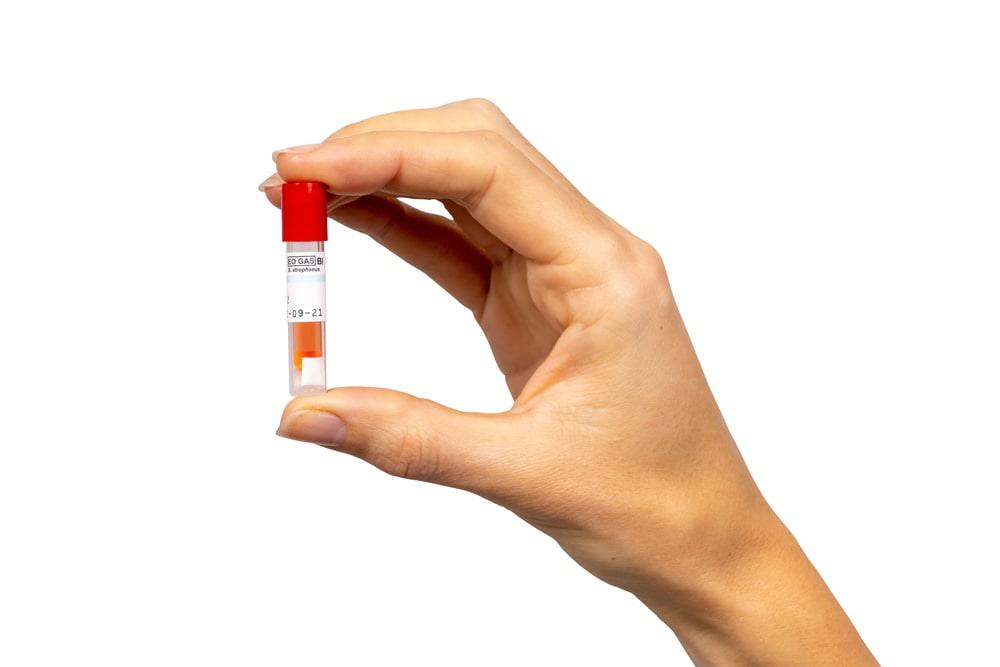
Our AN2203 BIs contain 106 B. athrophaeus bacterial spores on a filter-paper carrier packaged within a small, thermoplastic culture tube. Inside the thermoplastic culture tube is a sealed-glass ampoule of specially formulated soybean casein digest culture medium containing a color indicator which turns a dramatic yellow if spores grow. Color change reveals test results after 48 hours of incubation. Our new EOGas 4 – 6 hour sterilizer has a BI that incubates in just four hours. Operators place the BI in place hardest for gas to reach, usually between two items, unless the sterilizer has a PCD.
Our Process Challenge Device is integrated into the purge probe handle to ensure the AN2203 biological indicator is consistently placed in the most difficult location for EO to reach in every cycle – Inside a threaded metal screw-on cap. The PCD eliminates an extra layer of variability and human error and ensures that a negative BI result wasn’t given for those reasons. When the success or failure of a cycle could be a matter of life or death and the item being sterilized is complex, this level of assurance is simply necessary.
How Well do you Know your Chemical Steriliants?
Can you answer this simple quiz on the comparative toxicity of ethylene oxide, hydrogen peroxide and peracetic acid? How do they compare in terms of OSHA’s Permissible Exposure Limit (PEL) and NIOSH’s Immediately Dangerous to Life or Health (IDLH)?
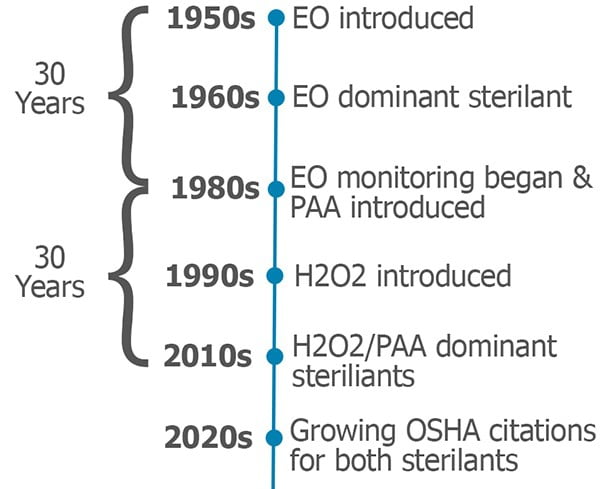
First some background: Ethylene oxide (EO) was introduced as a method of hospital sterilization in the 1950s. By the late 1960s, EO had become the dominant chemical sterilant in major healthcare facilities. OSHA was established in 1971 and, by the early ‘80s, it set permissible exposure levels for EO. Hospitals began environmental monitoring for EO and testing employees for exposure. So, it took about thirty years for the industry to recognize the potential dangers of EO.
Fast forward a few years. Peracetic acid (PAA) washer/disinfection systems were introduced in the late 1980s. Hydrogen peroxide (H2O2) sterilizers were introduced in the early ‘90s. From the introduction of these systems, there has been a widespread belief that H2O2 and PAA are safer than EO, and do not require exposure monitoring.

Comparative Toxicity Quiz
Which sterilant has the lowest Permissible Exposure Limit (PEL) AND Immediately Dangerous to Life or Health number (IDLH) – i.e. is the most potentially hazardous:
□ Ethylene Oxide
□ Hydrogen Peroxide
□ Peracetic Acid
Did you correctly answer EO and H2O2 have the same PEL of 1 ppm? Did you know the proposed PEL for PAA is even lower?
Regarding IDLH, the level for EO (800 ppm) is ten times higher than for H2O2 (75 ppm) – remember, a higher number represents lower risk. And for PAA, most people are surprised to learn that NIOSH is considering a proposed IDLH value that is lower still.
H2O2 and PAA systems have now been around for over thirty years, and they have become the dominate sterilization and disinfection methods in most healthcare facilities. If history repeats itself, the thirty-year grace period is coming to an end. Organizations governing worker safety have begun enforcing monitoring requirements for both H2O2 and PAA (Under the OSHA General Duty Clause, employers have an obligation to protect workers from serious and recognized workplace hazards even where there is no standard.)
Everyone who operates an EO sterilizer understands the importance of monitoring and testing. Are you testing for these other potentially hazardous chemicals in the workplace?
Answer: Peracetic Acid (proposed)
Is Disinfection Good Enough?
In the press you will often see the terms ‘disinfection’ and ‘sterilization’ used interchangeably. Most healthcare professionals know that one is better than the other, but when to sterilize a device versus disinfecting can be a matter of debate.
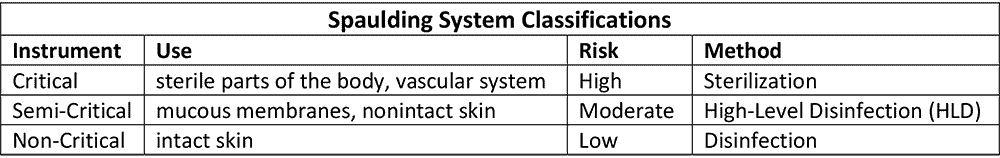
Over fifty years ago, microbiologist Dr. Earle H. Spaulding proposed a classification scheme to determine when re-usable medical devices should be disinfected or sterilized. His system was straightforward; three categories based on how an instrument was to be used (what part of the body it enters) and the subsequent risk of an infection.
The logic and simplicity of the system quickly brought it into widespread use. It was later adopted by the Centers for Disease Control and still forms the basis of international medical device disinfection guidelines today.
However, in the years since its introduction, the simplicity of Spaulding’s system has been challenged by our changing understanding of microbiology and micro-organisms. Biocide resistance, the role of biofilms and the increasing threat of multidrug-resistant organisms (MDROs) were not considerations in Spaulding’s original system and risk analysis. Modern medical instruments can be difficult to clean because of intricate device design (e.g., long narrow lumens, hinges), while delicate materials used in increasingly sophisticated instruments can be damaged by high temperatures and harsh chemicals.
The current debate over endoscope reprocessing is a good example of how Spaulding’s recommendations have grown complicated. Spaulding himself recommended that semi-critical devices (such as endoscopes) be sterilized – but noted that HLD could be used if sterilization was not practical or possible. Because sterilization processes take more time, most healthcare facilities chose to reprocess their scopes with HLD. In recent years, the increasing prevalence of infections linked to endoscopes and the growing virulence of MDROs, has fostered a sometimes heated debate among healthcare industry insiders and regulators.
A number of industry commentators, concerned about scope-linked infections, have been calling for the sterilization of all endoscopes. Many other industry leaders suggest that this is not necessary, as most scopes do not enter sterile areas of the body. Other endoscope experts are advocating for a phased approach, whereby a facility’s most sensitive endoscope models, such as duodenoscopes, are sterilized while others continue to be high-level disinfected.
This debate doesn’t just effect those in the proverbial ivory tower, the FDA recently opened an investigation into 450+ infection reports (including three deaths) related to reprocessing urological endoscopes. It may very well turn out, as it did during the deadly 2015 carbapenem-resistant Enterobacteriaceae (CRE) outbreak, that the endoscopes were properly reprocessed according to ‘Instructions For Use.’
It is time to pursue options that work. During the 2015 CRE outbreak, the FDA recommended four supplemental reprocessing measures. Subsequent field surveillance later confirmed ethylene oxide (EO) sterilization was the most effective of the supplemental measures, and validation studies showed that it was the only measure that assures the complete inactivation of highly resistant microorganisms. As you consider both patient safety and facility legal security – consider EO.
As one commentator put it, if an endoscope is going to be used on you or a family member, what would you prefer?
Hospital EO and the automobile – the race for gas efficiency
How are automobiles and ethylene oxide (EO) sterilizers similar? The answer may surprise you.
The post-World War II era saw a dramatic growth in automobile sales and broad adoption of EO sterilizers in US hospitals. In both cases, these early models were inefficient. In 1935 average fuel efficiency for American autos was around 14 miles per gallon (mpg) and that number dropped to only 12 mpg in the 1960s and early ‘70s (thanks, muscle car era!).1 Fuel efficiency simply was not valued during this period.
Likewise, early hospital EO systems were not concerned with gas efficiency. Ethylene oxide had been extensively tested in the 1930s and 1940s and was recognized as a promising sterilizing agent. The widespread introduction of plastics in the 1950s to medical instrumentation required a low temperature method of sterilization compatible with these new materials and EO fit the bill nicely.
Early hospital EO systems were typically fed by large 50lb tanks, kept on-site in specially ventilated storage rooms. Ethylene oxide is flammable and explosive in concentrations above 3%. Manufacturers of these tank systems reduced the potential danger by mixing EO with inert gases. The mixes, however, introduced their own problems – they were far less efficient and (unlike EO) the inert gasses used were discovered to be greenhouse gasses.
By the 1970s things began to change. The oil crisis of 1973 spurred a move toward automotive fuel efficiency that continues to this day.2 By 1980, average fuel economy for cars had reached 20 mpg. By 2008, it was over 25 mpg.3
For EO sterilizers, the impetus for change was the creation of OSHA in 1971 and a growing awareness of the potential dangers of chemicals in the workplace. The early 1980s saw the introduction of single-use cartridge based EO sterilizers. These systems, which used 100–170 grams of EO per cycle, represented a major step forward in gas efficiency and reduced risk over the tank-based systems.
Modern cars have an average fuel efficiency over 30 mpg with some hybrid models reaching near 60 mpg combined.4 EO has advanced even further. FDA recently cleared a new EO sterilization process that uses only 17.6g of EO per cycle – 90% less gas than any other system on the market.
Both technologies have continually innovated over the last 60 years. So just as you would not confuse a modern hybrid with the gas guzzlers of the ‘60s, do not confuse modern, high-efficiency EO sterilizers with the tank systems of yesteryear.Talk to you next month!
–Ted


Deeper Dive: Want to know more?
At Andersen, we are so proud of our EO – Flexible Chamber Technology (EO-FCT). It’s the proprietary technology that makes the astonishingly small, micro-dose of EO we use in our sterilizers so effective.

You see, we don’t sterilize “dead space.” A special sterilization liner bag encloses the items to be sterilized, the 17.6g gas cartridge and sterility indicators and that’s it. Any extra air is pulled out of the bag before the cartridge is activated.
We recently received several national awards for EO-FCT.
EO-FCT is also most likely one reason our upcoming EOGas 4 – 6 hour sterilizer recently achieved the ONLY FDA-clearance for terminal sterilization of duodenoscopes and colonoscopes – for lumens up to an 11.6 feet long and down to just 1.2mm in diameter! More Andersen FDA clearances.