HAW RIVER, N.C. – Andersen Sterilizers and its sister company, contract sterilization specialist Andersen Scientific, were awarded the very first master file in the FDA’s 510(k) Ethylene Oxide (EO) Sterility Change Master File Pilot Program.
“This master file award from the FDA makes it official, switching to Andersen from a traditional sterilization method just got even easier.” Tweet this
– A.E. (Ted) May
President & CEO
Andersen Scientific
As a result, 510(k) holders of devices labeled as sterile that wish to begin using Andersen Sterilizers’ EO-Flexible Chamber Technology (EO-FCT) systems to sterilize authorized class I or class II devices may reference the Andersen Sterilizers master file rather than submitting a new 510(k) for the sterilization change.
“We were awarded the FDA Innovation Challenge 2 for reducing ethylene oxide emissions with our proprietary technology three years ago,” said A.E. (Ted) May, Andersen Scientific President & CEO. “The FDA asked how they could help us as we further this mutual goal. We mentioned the hurdle (or perceived hurdle) for potential new customers worried that they will need to submit a new 510(k) if they switch from a traditional sterilization system to either Andersen Sterilizer’s EOGas 4 or EOGas 3 in-house systems or to Andersen Scientific’s contract sterilization services. This master file makes it official, switching to Andersen just got even easier.”
Now a medical device manufacturer considering a switch from a traditional sterilization method to Andersen’s ultra-efficient process, has this potential regulatory hurdle cleared for them. Whether they chose an in-house Andersen sterilizer or our commercial sterilization facility, they will not need to re-submit a 510(k) for their device.
There is a reason that ethylene oxide is used to sterilize the majority of this country’s new medical devices. It is incredibly effective against even the most dangerous pathogens, and it is compatible with a wide variety of materials. The beauty of the Andersen process is that it uses this very effective sterilant much more efficiently than any other method. Andersen sterilizers employ a flexible chamber system that uses 85-90% less gas than traditional EO sterilizers.
“We are very proud of this award and the completion of the FDA Master File. It is the next step in our history of relentless innovation,” said A.E. (Ted) May, Andersen Scientific President & CEO. “Ethylene oxide sterilization has been around since the 1960’s, and many people are not aware of how this technology has advanced. Andersen has obtained eighteen 510(k) clearances since 2015, including the first terminal sterilization clearance for duodenoscopes. This is a state-of-the-art system that delivers the proven reliability of EO in a modern, ultra-efficient process.”

Andersen Sterilizers sterilization systems use a proprietary flexible chamber, which requires less EO than established EO systems, supporting the FDA’s goal of minimizing the use of EO. Andersen Sterilizers designs and manufactures innovative tabletop and refrigerator-sized EO gas sterilizers for the manufacturing, research, medical and veterinary markets. Left: Andersen Sterilizers’ Anprolene AN75i, EOGas 4 and AN75j tabletop sterilizers.
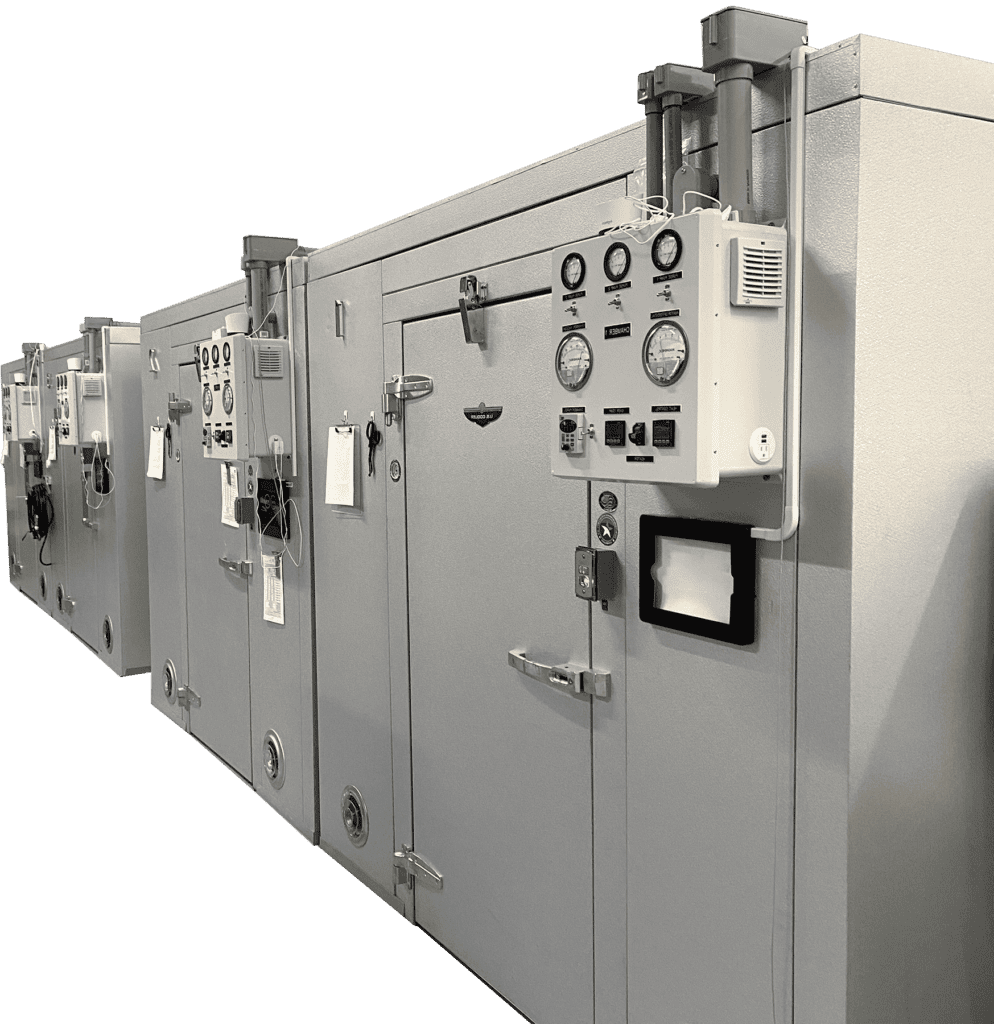
Andersen Scientific specializes in small- to medium-lot sterilization, custom cycles and fast turnaround. Right: Andersen Scientific’s state-of-the-art sterilization chambers.
Andersen Sterilizers designs and manufactures gas sterilizers for the veterinary, medical, research and manufacturing markets. American made, family-owned and trusted for over 60 years. Andersen Sterilizers headquarters is in Haw River, North Carolina, operates a UK office in Essex and partners with a select group of distributors around the world.
Andersen Scientific is so much more than a commercial sterilization facility focused on small to medium volume clients. The company is committed to exacting standards of quality and customer service, while serving customers as the “go-to” professionals knowledgeable in all matters relating to ethylene oxide sterilization. Andersen Scientific is quite possibly the most environmentally friendly ethylene oxide contract sterilization facility in the world.
Original article on Business Wire
Longer article on the topic from MD + DI (Medical Device and Diagnostic Industry)
