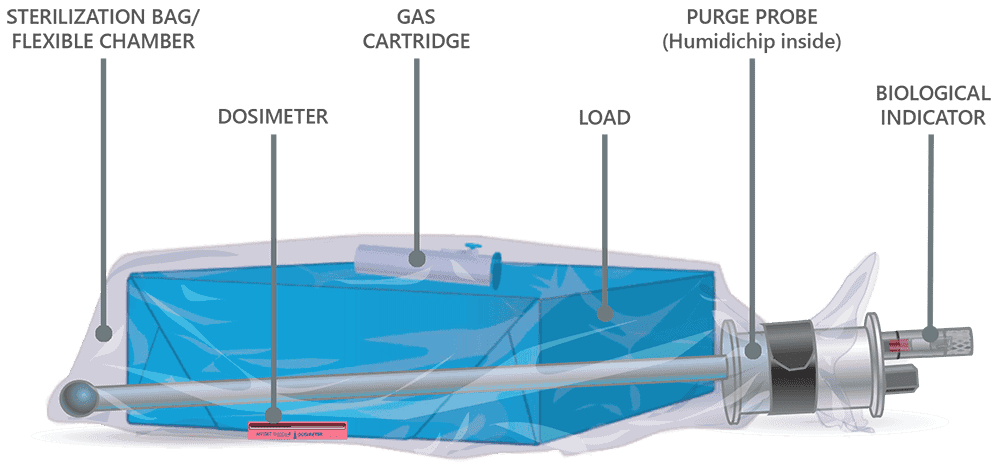
EO Sterilization: The FDA-recommended method for reprocessing flexible endoscopes
By Andersen Sterilizers
May 5, 2025
Share this post

Complex device reprocessing, superbugs and EO: A short history
Complex Devices that cannot be dependably disinfected:
Duodenoscopes are a type of upper gastrointestinal endoscope used during endoscopic retrograde cholangiopancreatography, or ERCP, to diagnose and treat disorders of the bile and pancreatic ducts.
Urological endoscopes are used for viewing and accessing the urinary tract.
Bronchoscopes may be used to examine, diagnose and treat disorders and diseases of the throat, larynx, trachea, and lower airways.
Which device will be next?
“Superbugs” are strains of bacteria, viruses, parasites and fungi that are resistant to most antibiotics and other medications commonly used to treat the infections they cause. These classes of antibiotics can include carbapenems – which are “last resort” antibiotics used to treat many types of serious infections caused by multidrug-resistant bacteria.1
Carbapenem-resistant Enterobacteriaceae – or CRE – are a particularly pernicious family of gram-negative bacteria resistant to these antibiotics. CRE and related superbugs have become a global public health scourge. The mortality rates for patients infected with CRE and certain other superbugs, particularly bloodstream infections, can be as high as 50% – or higher, in some patient subgroups.2,1
In February 2015, the FDA acknowledged for the first time, that duodenoscopes could remain persistently contaminated with life-threatening superbugs and related multidrug-resistant organisms.3 Examples of these potentially deadly microorganisms include CRE and carbapenem-resistant Pseudomonas aeruginosa.
During its subsequent review of several duodenoscope-related outbreaks, the FDA found that many of the superbug infections occurred despite staffers cleaning and disinfecting the duodenoscope in accordance with the manufacturer’s instructions.
According to the FDA, the complex physical design of duodenoscopes “may impede effective reprocessing,” ominously stressing that “meticulously cleaning duodenoscopes prior to high-level disinfection should reduce the risk of transmitting infection but may not entirely eliminate it.”3
This was a watershed finding because, previously, virtually every infection associated with a contaminated duodenoscope, or other type of flexible endoscope, had been attributed to a reprocessing breach – more specifically, to failure to clean and disinfect the device as instructed in its labeling. Reprocessing a flexible endoscope as prescribed by the manufacturer had always prevented cross-infection (with few exceptions), until that time first recognized in 2015.3
To combat the infection risk, enhance reprocessing and improve the safety of duodenoscopes, FDA advised healthcare facilities, in a safety communication published a few months later, in August 2015, to consider implementing one or more of four enhanced, or “supplemental,” measures, which included ethylene oxide sterilization.4
Drawing a distinction between disinfection and sterilization, FDA stated in this August communication “duodenoscopes should be subjected to high-level disinfection following manual cleaning after each use. When possible and practical, duodenoscopes should be sterilized due to the greater margin of safety provided by sterilization.”4
Indeed, subsequent field surveillance later confirmed ethylene oxide (EO) sterilization was the most effective of the supplemental measures, and validation studies showed that it was the only measure that assures the complete inactivation of highly resistant microorganisms.5
(Deadly) Alphabet Soup
MDROs: Multidrug-resistant microorganisms. Strains of bacteria, viruses, parasites and fungi that are resistant to most antibiotics and other medications commonly used to treat the infections they cause. Also known as superbugs.
CRE: Carbapenem-resistant Enterobacteriaceae, a particularly pernicious superbug.
HAI: Hospital-acquired infection or nosocomial infection. Most superbugs are acquired in a healthcare setting.
Common Superbugs

A continuing infection risk?
Some data suggests that the incidence of a duodenoscope transmitting microorganisms, including CRE and other potentially deadly superbugs, during ERCP decreased, at least for a time, following the FDA’s alerts in 2015 informing healthcare facilities and device manufacturers about this public health risk.6, 7
However, reports submitted to the FDA’s adverse event database for medical devices, or “MAUDE,” since 2015 indicate that, the number of FDA reports linking a duodenoscope to an infection apparently decreased in 2016 and even more so in 2017 compared to the relatively high number of cases reported to the FDA in 2015.6, 7 But 2018 reversed the trend and in 2019 the FDA received twice as many reports.
Ratcheting up the stakes, a report published in 2019 linked a duodenoscope to the possible transmission of a superbug carrying the mcr-1 gene, which can confer colistin antibiotic resistance to the microorganism.8 Like carbapenems, colistin may be used as a “last line of defense” for treating some types of multidrug-resistant infections. Notably, some colistin-resistant infections may be untreatable.
Several factors may explain these troubling trends, but one clear point warrants particular attention: the risk of infection associated with the use of a duodenoscope remains a concern today.
Indeed, according to the CDC, more healthcare-associated outbreaks have been linked to flexible endoscopes than to any other type of medical device.
CDC
FDA published alerts in 2019 and 2020 recommending that healthcare facilities and manufacturers “begin transitioning to duodenoscopes with disposable components” and, in the later alert, stressed “sterilization, particularly terminal (e.g., gas) sterilization, provides a greater margin of safety than high-level disinfection.”9
Andersen solved the problem
In 2015, Andersen Sterilizers had a duodenoscope clearance underway with the FDA. Because of the CRE outbreak, we were forced to withdraw our claim. At the time the agency wasn’t sure of the source of infections or how to test these complicated instruments post-sterilization.
We spent the next two years negotiating testing protocols with the FDA. During this time duodenoscopes were redefined as a “collection of instruments.” A new validation and simulated use testing protocol was chosen: The duodenoscope is inoculated at 7 of the hardest to reach sites in the scope with 106 bacterial spores.
This is a significantly higher bar than any previous FDA requirement. We are very proud to have achieved it.
Andersen validated duodenoscopes from all three major manufacturers and a colonoscope with an 11’6″ channel x 1.2mm!
Bronchoscopes and urological endoscopes pose a risk, too
Early in 2021, FDA acknowledged that other types of flexible endoscopes also remain a concern and may pose a risk of infecting patients with superbugs and related multidrug-resistant organisms.
In April, FDA issued a letter to raise awareness among healthcare providers about the risk of infections associated with reprocessed urological endoscopes, including cystoscopes, ureteroscopes, and cystourethroscopes.10
FDA wrote this April letter after acknowledging receipt of “over 450 MDRs describing post-procedure patient infections or other possible contamination issues associated with reprocessing these devices.”10
Similar to the circumstances surrounding the infections linked to “reprocessed” duodenoscopes, FDA concluded in the letter that both the reprocessing instructions and designs of urological endoscopes could contribute to these reported cases of infection and device contamination. Like the earlier alerts, the FDA advises facilities to follow IFUs, maintain their devices correctly and gives two reprocessing choices – one of which includes sterilization.10
The FDA later published a safety communication alerting the public to the risk of a third type of flexible endoscope ― reprocessed bronchoscopes ― similarly infecting patients with multidrug-resistant bacteria, again including CRE.11 The FDA’s notice provides updated advice that supplements the FDA’s earlier (and now outdated) alert, published in September 2015, also discussing infections linked to reprocessed (flexible) bronchoscopes.
This June alert focusing on bronchoscopes provided two new recommendations that were not discussed in the earlier 2015 communication: first, that healthcare facilities “consider using sterilization instead of high-level disinfection when feasible because sterilization has a greater safety margin than high-level disinfection.” Otherwise, if “sterilization is not available,” then high-level disinfection of bronchoscopes should be performed.11
Second, the FDA’s June alert recommends that healthcare facilities “not use damaged devices or those that have failed a leak test, as they could be a potential source of contamination.” Examples of endoscope damage include loose parts; damaged channel walls; and other signs of wear or damage.11
The FDA alert recommends considering single-use bronchoscopes for patients at higher risk of infection transmission, such as those who are immunocompromised or exposed to drug-resistant microbes. This guidance effectively creates a two-tiered standard of care based on patient risk, which can be problematic and ethically complex. Using ethylene oxide (EO) sterilization for reusable bronchoscopes could eliminate the need for differing standards. However, the guidance is vague about how to define or identify “increased risk,” raising concerns about subjective interpretation and the potential for undetected infections.
Growing support
The American Society for Gastrointestinal Endoscopy (ASGE) updated its “Multisociety guideline on reprocessing flexible GI endoscopes and accessories” in 2021 to state, “The use of EtO sterilization on duodenoscopes during infectious outbreaks has been associated with terminating these outbreaks, and such a modality should be considered in selected settings and patient populations.”14
The Multisociety guideline goes on to reference the work of Lawrence Muscarella, Ph.D., president of Principal at LFM Healthcare Solutions, LLC.
“EtO sterilization of duodenoscopes can be an effective tool in some clinical situations, specifically when there are infectious outbreaks observed among patients who have undergone ERCP. A systematic review by Muscarella examined all reported carbapenem-resistant Enterobacteriaceae–and multidrug-resistant organisms–related infections in the United States and Europe attributed to duodenoscope exposure and assessed the adequacy of reprocessing in these outbreaks.
Factors such as endoscope design, lapses in reprocessing guidelines, damage to the endoscope, or a lack of servicing, maintenance, and repair of the endoscope were hypothesized to be contributors to these outbreaks. In this review, 6 of 17 studies implemented EtO sterilization as an intervention during infectious outbreaks; in at least 3 and possibly 6 studies, this intervention yielded an absence of culture positivity from duodenoscopes at all sites, thereby stopping the outbreaks. Similar data exist for EtO sterilization ceasing outbreaks attributed to bronchoscopes. Therefore, the implementation of EtO sterilization appears to be an effective tool for terminating carbapenem-resistant Enterobacteriaceae and multidrug-resistant organism outbreaks associated with duodenoscopes.
The Multisociety guideline and the FDA’s 2015 document both highlight concerns about ethylene oxide (EtO) sterilization, citing high cost, inefficiency, limited availability, potential toxicity, and possible damage to endoscopes. However, they appear to reflect outdated EtO systems that are no longer representative of current technology. Andersen sterilizers offers a modern solution that is cost-effective, highly efficient, and gentle on instruments, with minimal risk to personnel and the environment. Our sterilizers provide a safe and practical alternative for facilities seeking reliable sterilization.
Conclusions
The risk of superbug infections associated with reprocessed duodenoscopes, bronchoscopes and urological endoscopes, among other types of flexible endoscopes, remains a concern today, warranting attention and review of the FDA’s recently published alerts, along with manufacturer instructions, to mitigate this risk.
Failure to properly reprocess a flexible endoscope has been directly linked to deadly infections of CRE, carbapenem-resistant P. aeruginosa and related superbugs.13 The mortality rates of superbug infections can be as high as 50%, or higher.11 Cleaning and disinfecting the endoscope correctly, in accordance with manufacturer instructions, significantly reduces the infection risk, but may not entirely eliminate it, according to the FDA. 3,12
The FDA has repeatedly (most recently in June) recommended ethylene oxide gas sterilization over high-level disinfection for reprocessing these endoscopes because of its “greater safety margin.”11 Numerous studies have documented that EO sterilization of endoscopes during infectious outbreaks has been associated with terminating these outbreaks.14
Andersen holds the ONLY FDA clearance for terminal sterilization for duodenoscopes and colonoscopes, with a maximum lumen length of 3530 mm (11.6 feet) and minimum lumen diameter of 1.2 mm (the thickness of a Blu-Ray, less than the height of a dime).
We also hold FDA clearances for urological endoscopes and bronchoscopes, including: video bronchoscopes, cystoscopes, ureteroscopes, choledochoscopes, video gastroscopes and gastrointestinal videoscopes.
Recap
- EO is the FDA-recommended method for reprocessing flexible endoscopes because of its greater margin of safety.
- Andersen holds recent (post-2015) FDA clearances for flexible endoscopes in all troublesome categories. In some cases, we hold the only clearance.
- We hold clearances for chemical and biological indicators and packaging that maintain the sterility of endoscopes for 3-6 months after reprocessing.
- Our sterilizers are tabletop (two larger models available). They are easy to install and use – with free training for the life of your sterilizer. An Andersen Sterilizer is a seamless addition to your practice.
Is it time to protect your patients and safeguard your business?
Spread the Word

Andersen Sterilizers
May 5, 2025
BLOG
Latest Insights on Sterilization
Get expert updates on safety, compliance, and innovation.
Why Hospitals and Labs Trust EOGas 4PLUS for Reliable Sterilization
Ethylene Oxide Gas Sterilizer Risk vs. Reward
Low Temperature. Low Dose. Low Emissions.
Expert Guidance for Your Needs
Tell us about your sterilization requirements — our experts are here to guide you to the perfect EO solution.