Innovation
Breakthrough Technology Revolutionizing EO Sterilization
Andersen’s EO-Flexible Chamber Technology (EO-FCT) represents a groundbreaking approach to gas sterilization. This method not only enhances efficiency but also ensures a gentler sterilization process at lower temperatures.

Our award-winning Ethylene Oxide-Flexible Chamber Technology (EO-FCT) is what sets us apart from our competitors. We have FDA Clearances on multiple ethylene oxide sterilization systems. Our process is precise, reliable and proven.
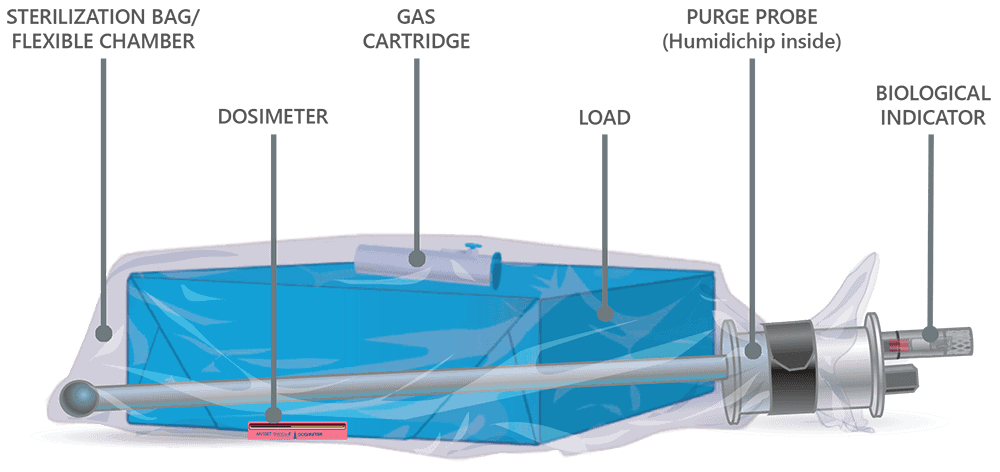
How EO-FCT works
Our proprietary EO-Flexible Chamber Technology process is a unique sterilization method shared by the Andersen Anprolene AN 75 series and EOGas 4 series models. The above graphic illustrates a loaded sterilization bag, flexible chamber, secured with a hook-and-loop strap around a purge probe.
Air Purge
The sterilization cycle begins by purging excess air from the sterilization bag, the Flexible Chamber. Air molecules are exhausted from the sterilization bag through the purge probe and purge tube. With excess air removed, the ethylene oxide concentration in the sterilization bag is maximized during sterilization, thereby minimizing the amount of EO needed for effective sterilization.
Cartridge Activation and Sterilization
Once the initial purge is complete, the gas cartridge is manually activated by pressing the button on the cartridge through the wall of the sterilization bag. Ethylene oxide spreads throughout the sterilization bag and permeates the items being processed. The gas-impermeable flexible chamber maintains a high gas concentration during the sterilization cycle.
Gas Ventilation and Aeration Cycles
At the end of the sterilization cycle, the ventilation portion of the sterilization bag begins; 30 minutes of ventilation after the 3-hour gas exposure and one hour after the 6-hour gas exposure. A valve in the purge probe opens, allowing fresh air to travel down the probe and into the back of the bag. Ethylene oxide is exhausted through the purge probe.
Aeration continues after ventilation, flushing air through the sterilization bag and out the purge tube. Aeration of the devices in the sterilization bag ends when the operator exits the cycle to remove the sterilization bag from the sterilizer.
Terminal Sterility
Achieves FDA-required 10-6 sterility assurance level (SAL) for terminal sterilization of medical devices.
Easy Installation
Easiest in the industry; requires only a dedicated electrical outlet and an exhaust line. See your sterilizer’s specific specs for full details.
Micro-dose
Terminal sterility (10-6 SAL) with 90% less EO than any other system on the market. EOGas 3 sterilizers use even less per cycle.
Zero Emissions
Andersen’s optional emissions abator is a simple, cartridge-based system that employs a dry catalyst resin. The resin converts ethylene oxide to biodegradable organic compounds. Replaceable cartridges are non-hazardous.
Smart Cabinet Design
A ventilation port in the back of the cabinet actively draws fresh air from the room through the cabinet and out the back exhaust through the entire cycle. This process ensures airflow in one direction and significantly mitigates risks.
Gentle
Achieve 10-6 SAL without those damaging downsides: high heat, steam, deep vacuum, oxidation or corrosion. Our process is damage-free.
Unmatched Compatibility
Sterilizes your most delicate instruments, electronics, drills, fiber optics, handpieces and cameras. As well as those plastic, fabric, cellulose or rubber items that you can’t sterilize with other methods. Most items except for food, drugs or liquids can be sterilized.
Right-sized
We have models from tabletop to refrigerator sized. Most designs fit conveniently on a countertop or table.
Active Aeration
Andersen’s purge probe flushes the sterilization bag with a constant flow of fresh air at the end of the cycle – no need to transfer items to a separate area. Active aeration continues indefinitely until products are removed.
Discover the key benefits of our advanced sterilization technology.
Affordable Solutions for Every Practice
Our units are economically priced, ensuring a quick return on investment.

User-Friendly Operation for All Staff
Set it and forget it – our system is incredibly easy to use.
Comprehensive Training for Your Team
We provide free training for your staff, ensuring effective operation.
Impact
Environmental Impact
EO-FCT enables us to use just a microdose of EO. This means we are also naturally emitting far less.
We also offer optional emission abators. The simple, cartridge-based system employs a dry catalyst resin that converts ethylene oxide to biodegradable organic compounds. Replacement cartridges remove more than 99% of the EO in the exhaust stream, resulting in a fraction of a gram of total EO emissions throughout a multi-hour cycle. This tiny amount of EO is vented to the outside, where it disperses rapidly, quickly becoming undetectable, making the process zero emission.
While traditional contract sterilization facilities can emit 1 or more tons of EO every year, even our worst-case calculation (running 24 hours a day, 365 a year, without stopping even for aeration), our systems emit 24 to 96.5 lbs. of EO per year. With an abator, those numbers become .24 to .97 lbs.
Two National-Level Regulatory Environmental Awards
In 2019, Andersen was selected by the Food and Drug Administration (FDA) to help develop strategies or technologies to reduce EO emissions to as close to zero as possible. This FDA Innovation Challenge collaboration is ongoing.
In 2020, Andersen received the Small Business Environmental Stewardship Award for its high-efficiency, low-emission sterilization process and its cooperation with State and Federal officials on regulatory and permitting issues.
2019 FDA Innovation Challenge 2 Winner
High-Efficiency EO Sterilization Process
2020 SBA Environmental Stewardship Award
Zero Emission Technology
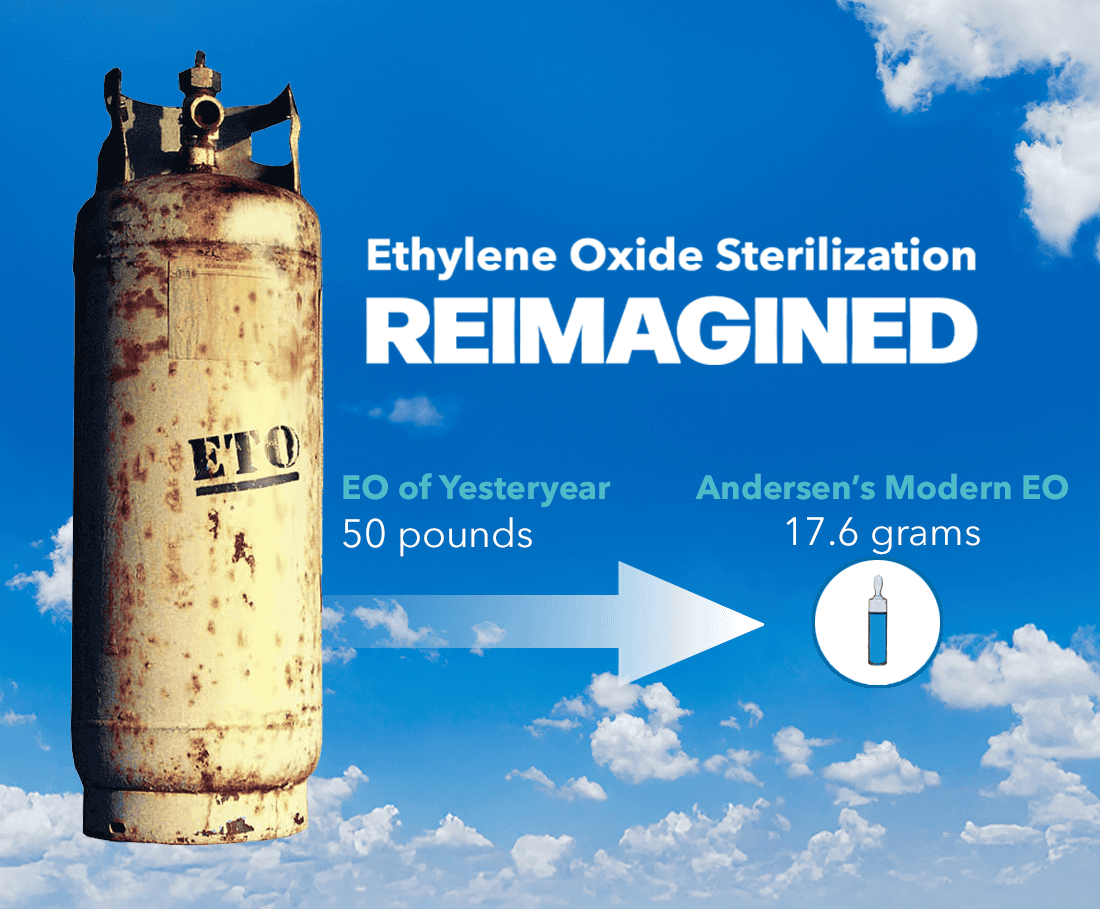
EO of Yersteryear
Hospital Sterilization
Skip to: Contract Sterilization
Ethylene oxide was extensively tested by U.S. government labs in the 1930s and 1940s. EO was recognized as a promising sterilizing agent. In the 1950s, as medical devices were being manufactured with plastics, hospital facilities required a method for low-temperature sterilization. Low-temperature sterilization systems were developed, and by the 1960s, EO became the dominant sterilization method in U.S. healthcare facilities.
These early hospital EO sterilizers were a breakthrough in low-temperature sterilization, but they were not without problems.
Environmental Concerns: Ethylene oxide is flammable and explosive in concentrations above 3%. Manufacturers of these tank systems reduced the potential danger by mixing EO with inert gases. The mixes, however, introduced their own problems – they were far less efficient and (unlike 100% EO) the inert gases used were discovered to be greenhouse gases. These EO mixture systems were scheduled for phase-out in the early 1990s and were taken off the market in 2012.
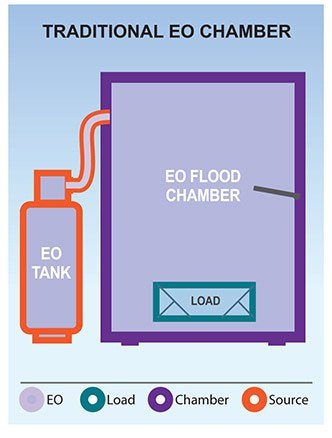
Large tanks of gas: Early hospital EO systems were typically fed by large external 50lb tanks. These systems used pounds of EO per cycle, and supplies of additional large tanks required storage in specially ventilated rooms.
Wasted Space: Besides the storage room for EO tanks, these early systems required external air compressors and fixed water lines. EO sterilizers of this era were built into wall units with a service room behind the sterilizers. The EO sterilizers themselves were installed in separate rooms to prevent EO from entering the general workspace (see illustration). This extra room meant hospitals had to dedicate a large amount of space to the installation and operation of their EO sterilizers.
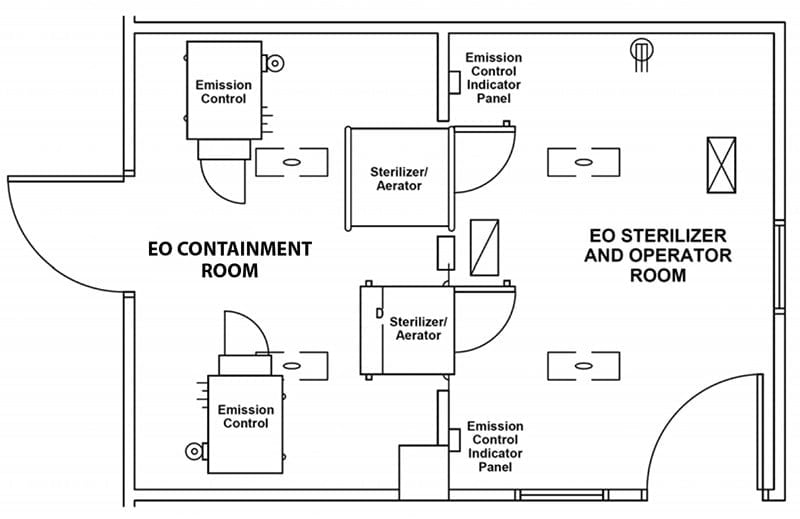
While EO is an extremely effective sterilant with the widest range of material compatibility, the early traditional EO chamber systems had some significant drawbacks. In the mid-1990s, Hospitals began to move away from EO and toward other options such as H2O2 and PPA. We discuss how EO compares to H2O2 and PPA here.
Sterilization
Contract Sterilization
Traditional contract EO sterilization facilities sterilize medical devices by the pallet load. Many current EO “pallet chambers” can sterilize over 50 pallets of product at once. EO pallet chambers are the typical method for sterilizing most new medical devices, especially products that are produced in large quantities.
However, these large EO contract facilities may not be the best option for manufacturers of specialty devices that are produced in smaller volumes. Likewise, firms that are developing new devices and require a fast turnaround of small numbers of devices require a different solution.
Gas usage is also an issue. A typical EO contract sterilization facility uses between 6 to 8 lbs. of EO to sterilize a single pallet of devices. In contrast, our Andersen contract option with a modified EO-FCT system uses just .9 to 1.6 lbs. to process the same volume of product. That’s a 77% to 87% reduction of EO consumption.
All of this seems so much more relevant when we speak in terms of the bottom line. With Andersen (in-house or contract) you are able to reduce turnaround time, better manage your inventory and maintain control over your product. Contact us to discuss whether Andersen contract sterilization or in-house processing is right for you.
Please also see the Environmental Impact tab.
Expert Guidance for Your Needs
Tell us about your sterilization requirements — our experts are here to guide you to the perfect EO solution.
