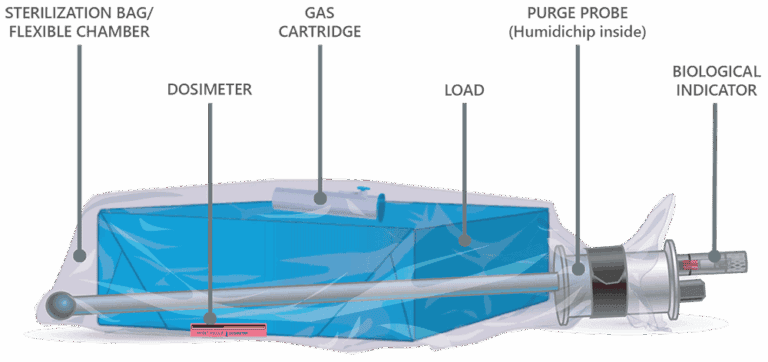
Low Temperature. Low Dose. Low Emissions.
Never has peace of mind required so little investment. Andersen’s breakthrough technology is revolutionizing ethylene oxide (EO) sterilization. Equipped with flexible chamber technology, the newly released EOGas 4PLUS requires a fraction of the amount of ethylene oxide used by its rigid-chamber competitors. This modern-day approach to gas sterilization not only enhances efficiency but also ensures…