Sterilization Solutions For Manufacturing
Andersen provides reliable terminal sterilization solutions tailored for various industries. Our products are competitively priced, user-friendly, and designed for maximum versatility.
Manufacturing
Achieve Terminal Sterilization
For more than six decades, Andersen Sterilizers has focused on just one thing —
the design and manufacturing of effective and affordable low-temperature
sterilization for healthcare, veterinary, research and manufacturing markets.
Sterilization game-changer
No matter where you are in the product development process, from R&D to production, we have in-house and contract sterilization solutions to fit your needs. Achieve terminal sterility with unparalleled compatibility.

Make Sterilization Part of Your Production Process
- Require sterile processing of items in smaller lots
- You have items or devices that are sensitive to heat or moisture
- Have a wide variety of items to sterilize (EO is the most versatile method available)
- Need to sterilize frequently
Benefits of in-house sterilization with an Andersen sterilization system:
- Speed up the pace of research with on-site sterilization
- Mitigate contamination concerns by keeping samples in the lab
- May be installed in areas with a small available footprint
- Feature easy, inexpensive installation
- No tanks, external compressors or fixed water lines
- Affordable – essential equipment in every wet lab
- Are self-contained units – The sterilization bag acts as the chamber, and the cabinet acts as the EO containment area or vent hood
- Easy to use and include free training by our experienced staff, who have decades of experience
- Control every aspect of your sterilization workflow and improve your ability to manage inventory
- Reduced sterilization turnaround time
- Reduced transportation costs and environmental impact
- Allowed in municipalities where other gas sterilizers are not, due to Andersen’s ultra-low emissions

State-of-the-Art Facility Offering Custom Cycles and Fast Turnaround
Consider contract sterilization at Andersen’s award-winning, FDA-registered and ISO-approved facility (Andersen Scientific) if you:
- Cannot sterilize in-house (larger items or too much)
- Require new custom cycle development and/or validation
- Need sterilization testing of new devices
- Need package validations for sealers, pouches and other packaging systems
Opting for Andersen contract sterilization offers these benefits:
- In-depth R&D experience relative to sterilization
- Industry experts – Andersen representatives are longstanding members of the AAMI and ISO committees that create the standards used in the industry
- Rapid turnaround: devices/products can be sterilized and shipped back out within time frames:
- 24 to 48 hours
- Can customize cycles to meet your specific requirements
Services:
- Validation
- Sterilization process
- Contract sterilization
- Lab services
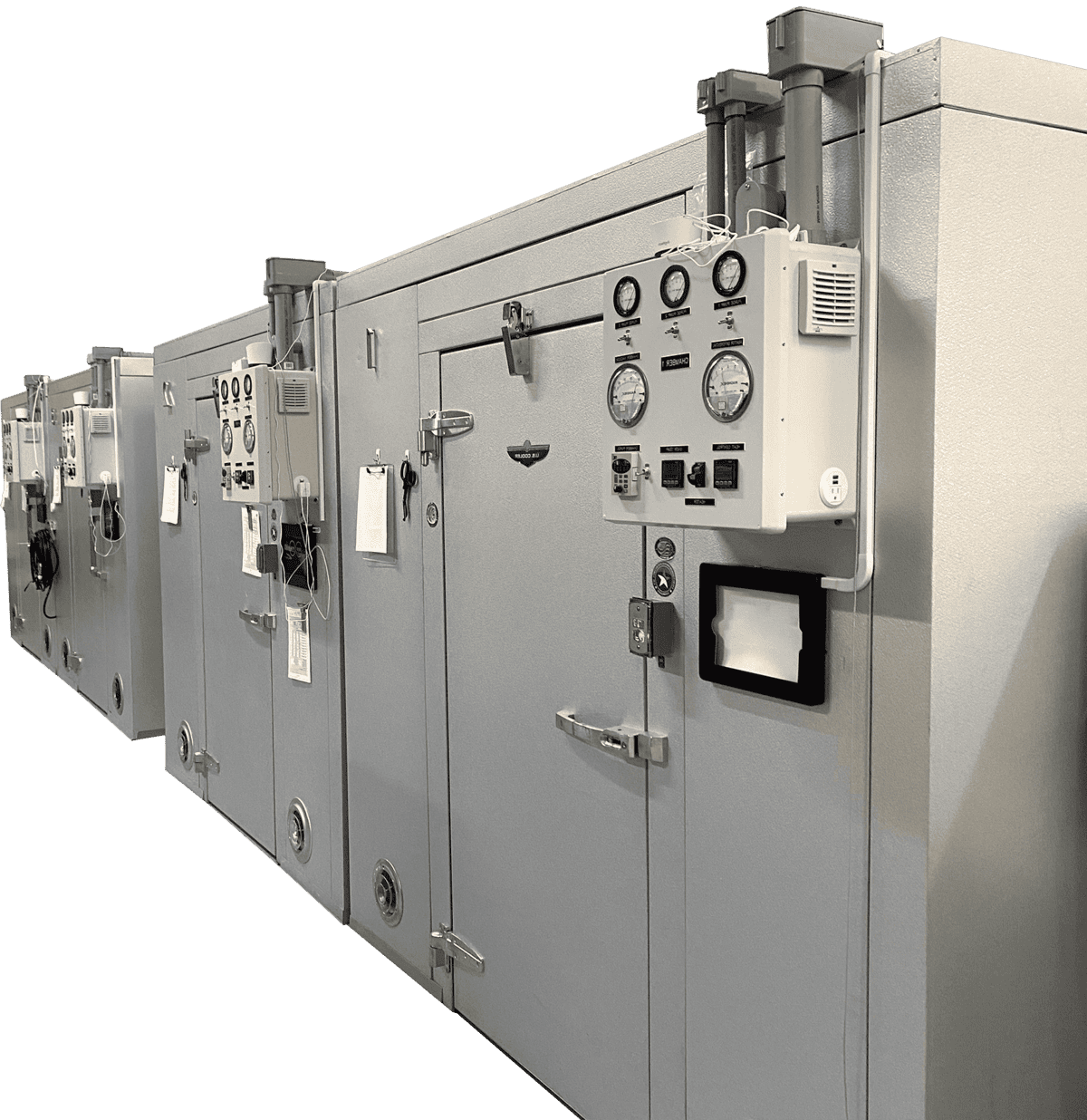
FEATURE PRODUCTS
Three in-house sterilizer line choices
Choose the best sterilizer for your needs.
WHY CHOOSE ANDERSEN
Discover the Advantages of Choosing Andersen for Your Research Needs
From FDA-clearances to award-winning EO technology, our sterilizers deliver reliability, safety, and efficiency for your research and laboratory needs.
Terminal Sterilization
Achieves FDA-required 10-6 sterility assurance level (SAL) for terminal sterilization of medical devices. In fact, we have 15 recent FDA clearances.
Small Footprint
High capacity, small footprint. Sterilize a multiple loads without taking up space. From tabletop to refrigerator-sized – Andersen has a unit to fit your needs.
Award-winning
Our exclusive EO-Flexible Chamber Technology and the resulting ability to use a microdose of EO has garnered two national awards.
Scalable
Our in-house systems allow you to expand incrementally and virtually indefinitely based on your needs. You can also expand use of our contract option as needed.
Flexible
With Andersen you can choose in-house sterilization, contract or both. Any way you slice it, Andersen makes it so easy.
Control
Oversee every aspect of your sterilization workflow and improve your ability to manage inventory.
Case Study
Contract manufacturing case study
Challenges:
When the COVID-19 pandemic hit, authorities began requesting testing supplies and Keystone Solutions Group knew they could help. From first request to sending out swabs – it was just four weeks.
Solutions:
“Andersen Scientific was willing to jump in and understood how to get there,” said David Furchak, Launch Architect at Keystone. Andersen expedited the validation and packaging of Keystone’s swabs starting in March 2020. “The employees at Andersen have been good about figuring out the best path forward. Their collaboration and willingness to have conference calls at all hours is the key to success.”
Results:
Andersen sterilized two pallets per week from Keystone for over four months – during the peek of the pandemic. Generally, we were able to return the swabs the day after receiving them.


BLOG
Latest Insights on Sterilization
Get expert updates on safety, compliance, and innovation.
Veterinarians Trust Andersen Sterilizers
Why Hospitals and Labs Trust EOGas 4PLUS for Reliable Sterilization
How Andersen EO Sterilizers Help Veterinary Clinics Save Money
Expert Guidance for Your Needs
Tell us about your sterilization requirements — our experts are here to guide you to the perfect EO solution.





