Healthcare
Protecting Patients
For more than six decades, Andersen Sterilizers has focused on just one thing —
the design and manufacturing of effective and affordable low-temperature
sterilization solutions for healthcare, veterinary, research and manufacturing markets.
We help you protect your patients and preserve your instruments.
Is HLD Enough?
Hospital-acquired infections not only put your patients at risk, but also put practices at risk. They have been reported as recently as fall 2024
Responding to a 2019 outbreak, in 2020 FDA disseminated an alert that sterilization, particularly terminal (e.g., gas) sterilization, provides a greater margin of safety than high-level disinfection.
Andersen’s FDA-cleared EOGas 4PLUS provides terminal sterilization for a wide range of medical devices, including those with complex lumens that can degrade using other modalities, such as steam and H2O2. FDA-cleared, EOGas 4PLUS is the only system proven to sterilize the longest lumens.
Commonly Sterilized Items
Andersen systems deliver terminal sterilization (10⁻⁶ SAL) without compromising device integrity — no dulling, clouding, or degradation. Commonly sterilized devices include:
- Flexible endoscopes (including the longest lumens)
- Hand-pieces and cords
- Mix-material instruments or trays
- Implants, sharps, plastics, rubber, non-wovens/cellulose, fiber optics, electronics and batteries
Learn more about how Andersen’s technology can help prevent hospital-acquired infections:
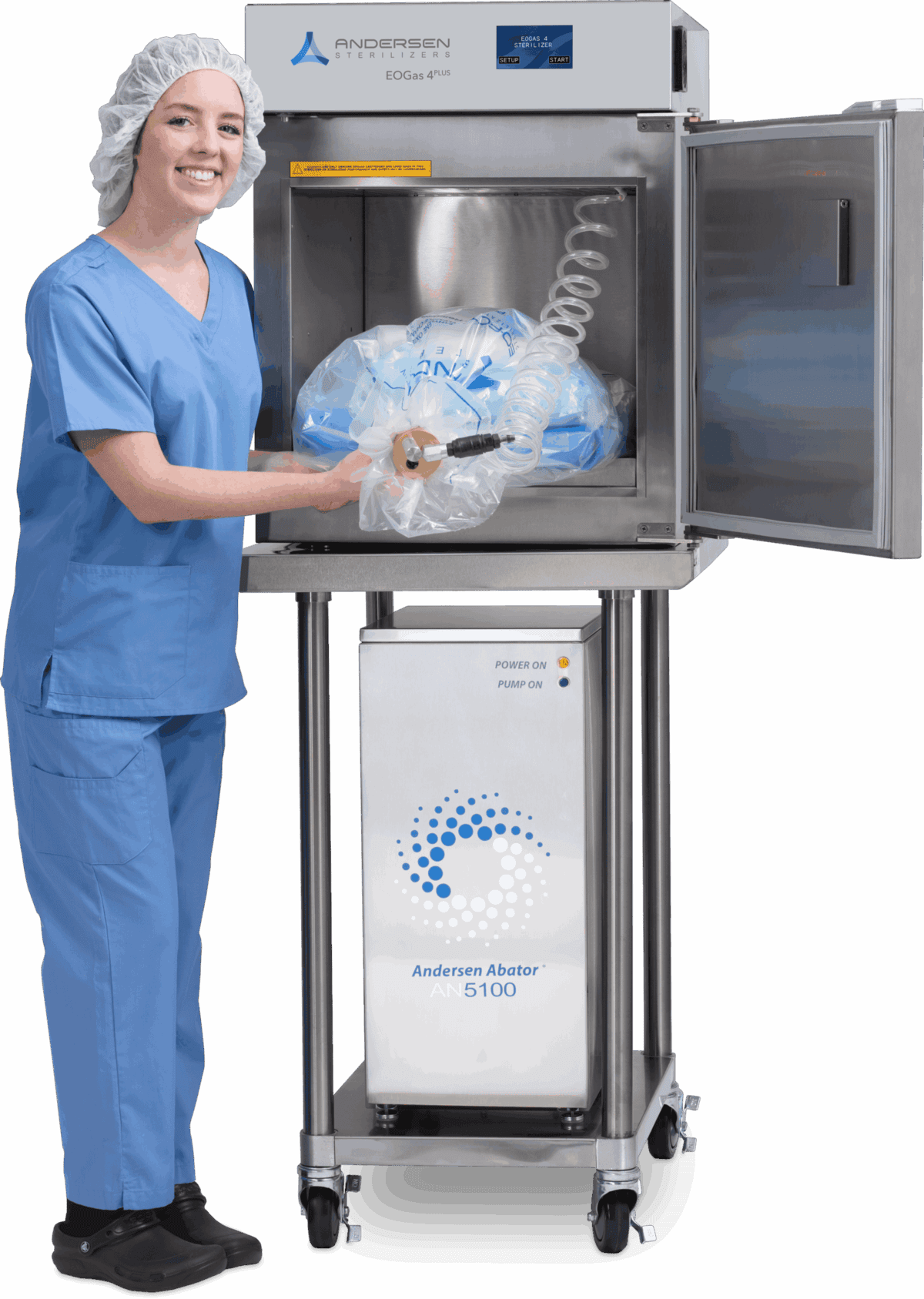
FEATURE PRODUCT
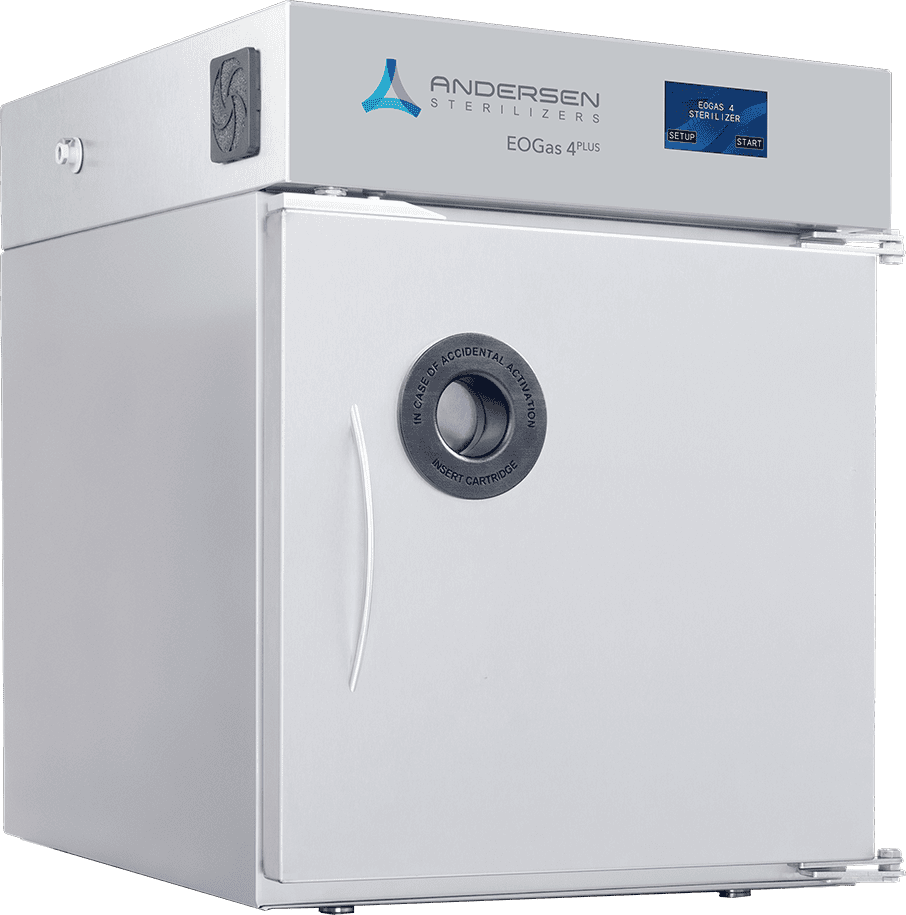
EOGas 4PLUS

Best for Healthcare & Research
EOGas 4PLUS
The newly launched EOGas 4PLUS sets a new industry standard for reprocessing difficult-to-sterilize devices, including complex duodenoscopes and colonoscopes. Featuring our award-winning EO-Flexible Chamber Technology, EOGas 4PLUS provides safe, effective sterilization with ZERO emissions when used with an Andersen abator.
Image Gallery
Explore the EOGas 4PLUS
Accessories
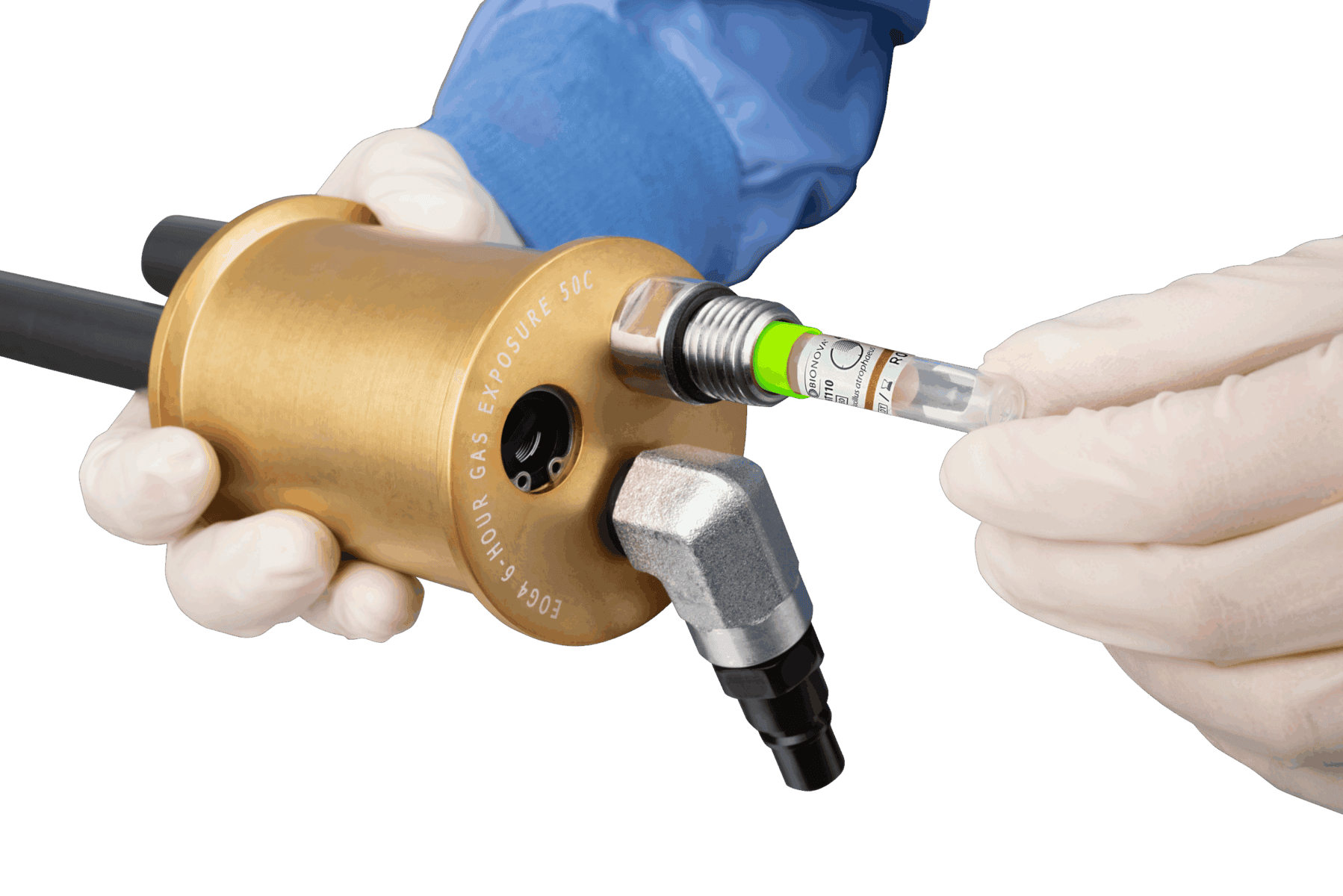
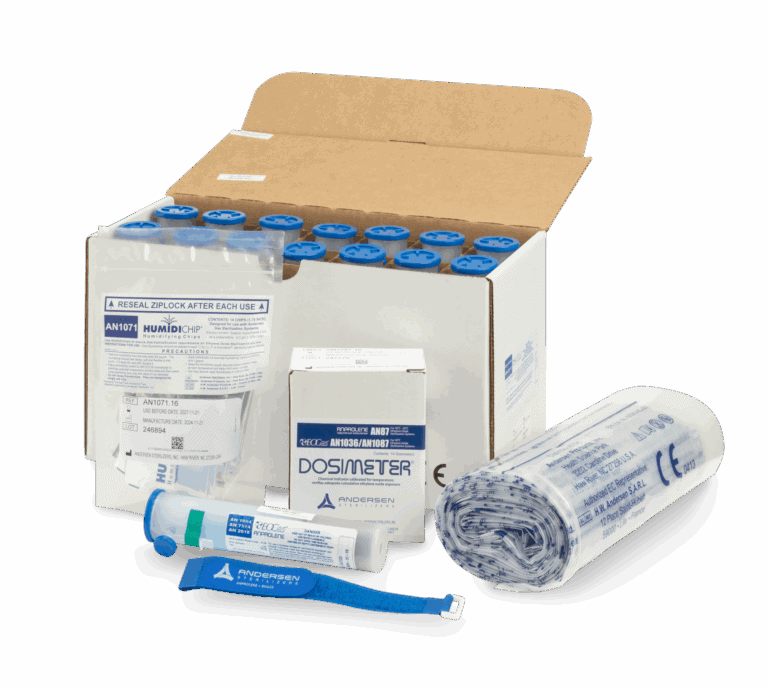
The EOGas 4PLUS Refill Kit from Andersen Sterilizers includes 17.6-gram cartridges, sterilization bags, Humidichips, and Dosimeters for 14 cycles. Designed for low-temperature sterilization, this kit ensures safety and efficacy for medical devices in healthcare and veterinary settings. Take a moment to explore the full complement of EOGas 4PLUS accessories and supplies.
AN1004.16 EOGas 4 Refill Kit
AN2203 EZTest Biological Indicator
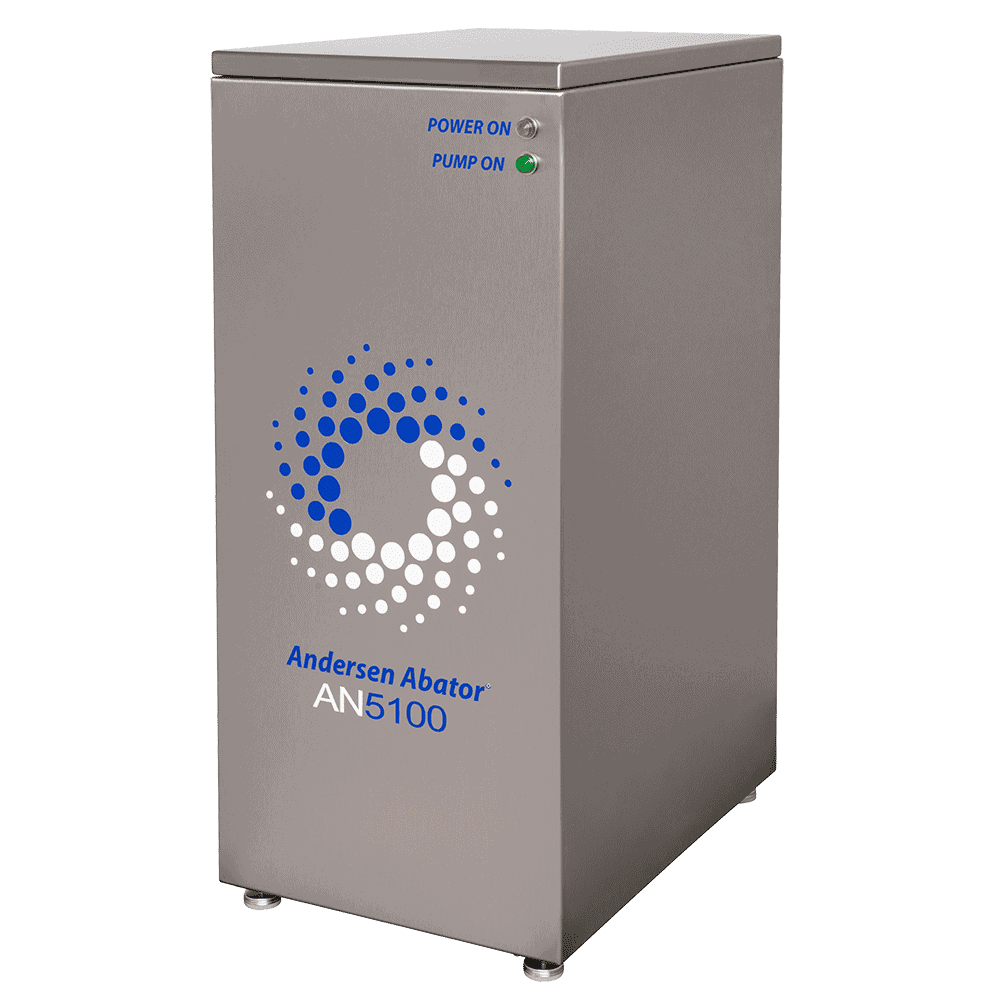
AN5100.01 EO Abator
Downloadable Documents
User Manuals and Technical Documents for EOGas 4PLUS
Installation Instructions
EOGas 4PLUS Brochure
EOGas 4 Zero Emissions Math
English
Ethylene Oxide Safety Data Sheet – EO SDS
Compare All Andersen Sterilizer Models
English
Health Canada License EOGas 4
English
Health Canada License AN1004
English
Instructions for Use: EOGas 4
English
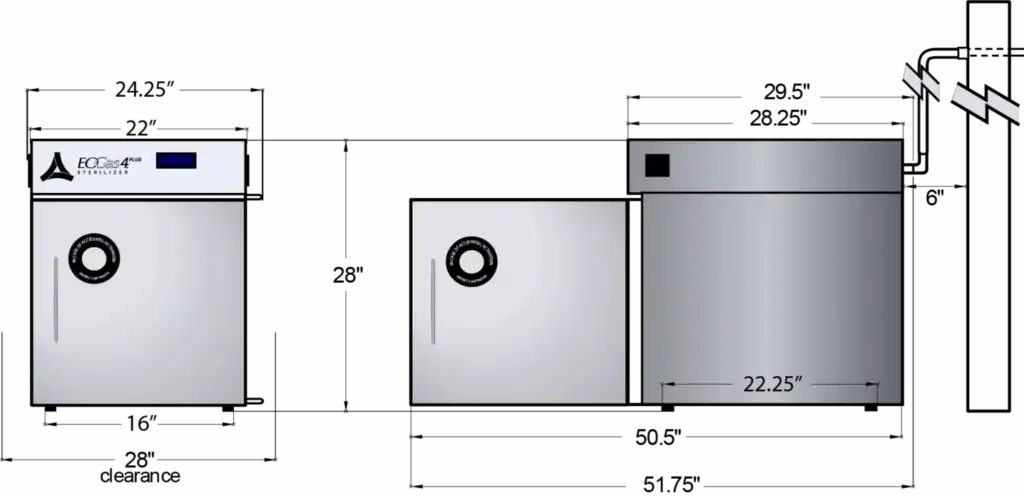
SPECS – Exterior Dimensions. See the brochure for interior dimensions.
Best for Veterinary
Free Lifetime Training for All Operators
Andersen provides free training for as many operators as required, for the lifetime of any of its sterilizers.
Videos
Coming soon! For more information, Contact Us.
SOLUTIONS
Find Your Ideal Sterilization Solution
Not sure whether to invest in an in-house sterilizer or outsource to a contract facility? Answer these quick questions to discover which solution fits your workflow, budget, and sterilization needs.
WHY CHOOSE ANDERSEN
Discover the Advantages of Choosing Andersen for Your Research Needs
From FDA-clearances to award-winning EO technology, our sterilizers deliver reliability, safety, and efficiency for your research and laboratory needs.
FDA Clearances
Our sterilizers are FDA-cleared for medical settings. We have 18 clearances covering everything from the sterilization of the longest and narrowest colonoscope lumens.
Size Matters
Our space-saving tabletop designs enable even the smallest practices to have access to sterilization solutions that ensure their patients are protected.
Eliminate Superbugs
Hospital-acquired infections not only put your patients at risk, but also put practices at risk. With incidents on the rise, sterility is no longer a luxury but a simple necessity.
Abatement Option
We are proud to offer dry catalyst resin abators. The resin converts ethylene oxide to biodegradable organic compounds. This contrasts catalytic abators that burn off EO and can be expensive to install.
Time
When you think about staff time management, don’t just consider sterilization time, also consider capacity. Instead of running all day, staff can run one sterilization cycle overnight.
Operator Safety
Our exclusive technology works behind the scenes to keep your staff safe. The microdose of EO and smart cabinet design are just two of these safeguards.
Why Ethylene Oxide?
There are many benefits to ethylene oxide sterilization, some of which may be crucial to your hospital or clinic, especially when reprocessing complex multi-channel endoscopes and delicate medical devices. Andersen’s FDA-cleared sterilizers offer several advantages, including low per-cycle cost, low-temperature operation and EO-Flexible Chamber Technology, which allows our systems to use a microdose of EO — just 17.6 grams per cycle. Our systems are able to achieve terminal sterilization (10-6 SAL) with 90% less EO than our competitors — this is ethylene oxide sterilization reimagined.
Customer Testimonials
Our experience with Andersen has been outstanding.
BLOG
Latest Insights on Sterilization
Get expert updates on safety, compliance, and innovation.
Don’t Allow Your SPD To Become A Statistic
Sterilization for Medical Devices
Veterinarians Trust Andersen Sterilizers
Expert Guidance for Your Needs
Tell us about your sterilization requirements — our experts are here to guide you to the perfect EO solution.