How does Andersen’s Ethylene Oxide – Flexible Chamber Technology (EO-FCT) sterilization process work?
EO-Flexible Chamber Technology Sets Us Apart
Ethylene Oxide-Flexible Chamber Technology® (EO-FCT) is Andersen’s proprietary and award-winning sterilization process. It is shared by the Andersen Anprolene® and EOGas 4Plus® series models. EO-FCT allows our sterilizers to use almost 90% less gas per cycle than any other system in the world.
Newly released EOGas 4PLUS, featuring EO-FCT, is proven to sterilize the longest lumens on the market using just a microdose of EO — 17.6 grams per cycle.

Load Preparation
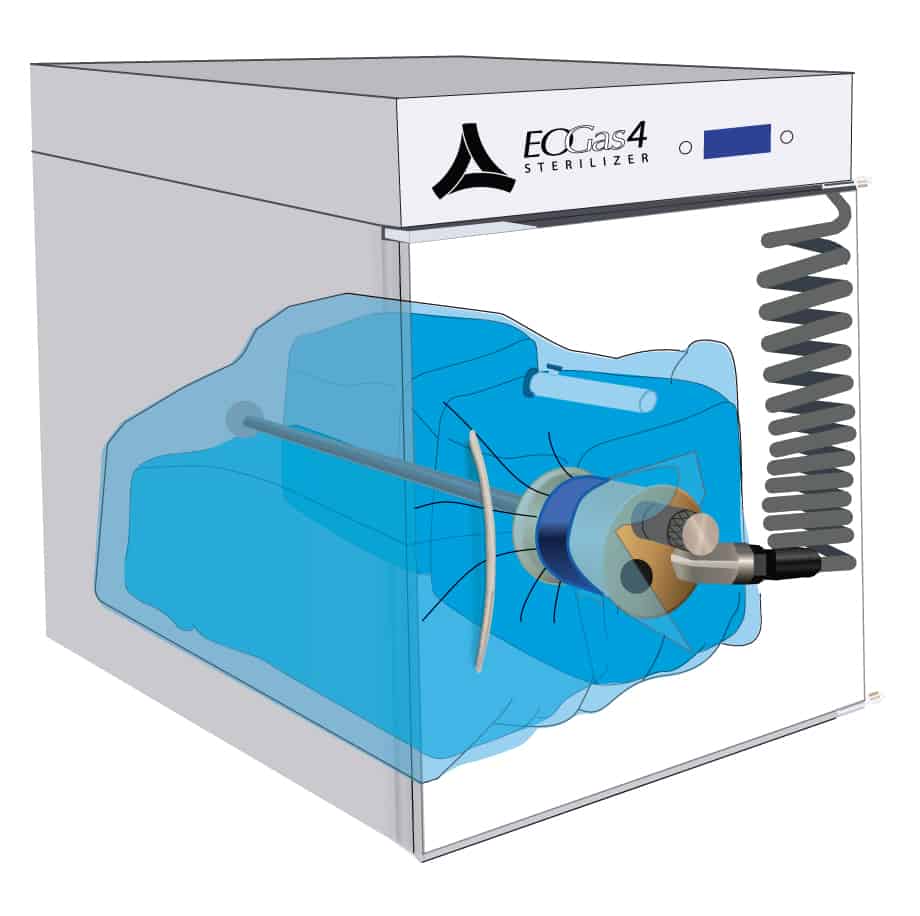
The EO-FCT process is a unique sterilization method shared by the Andersen Anprolene and EOGas 4 models.
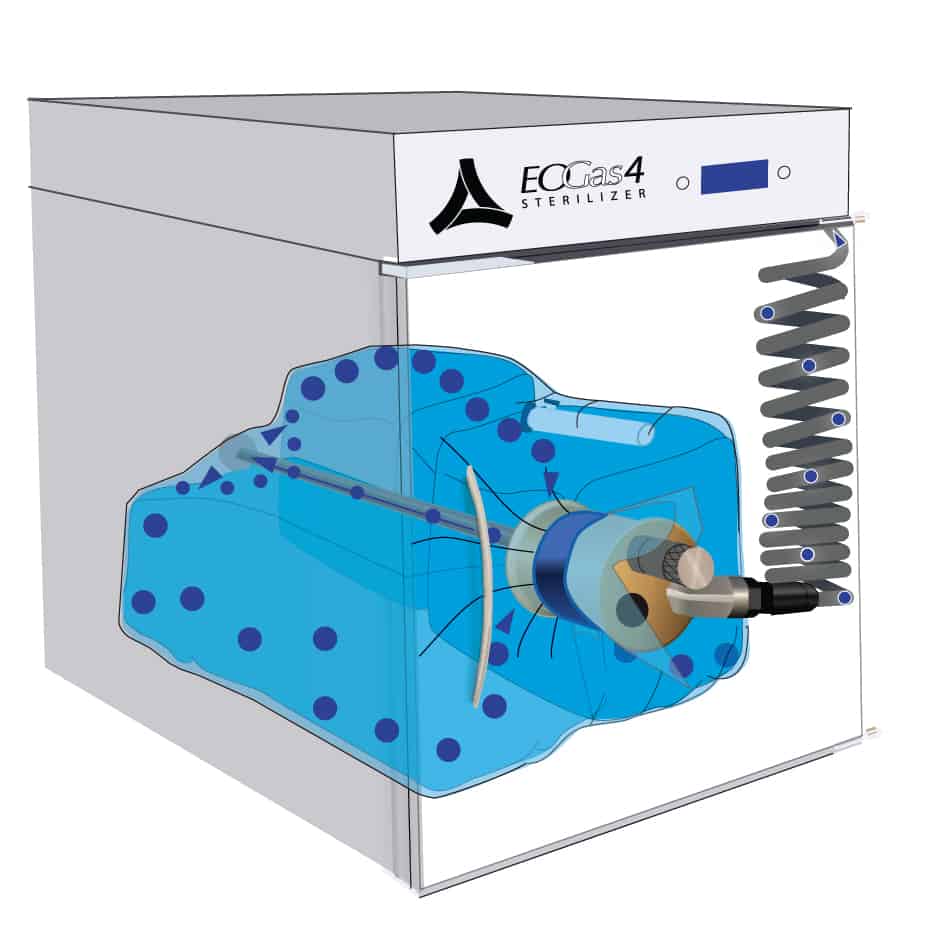
This cut-away view of a loaded Andersen sterilizer reveals a loaded sterilization bag—flexible chamber—secured with a hook-and-loop strap around a purge probe.
Air Purge
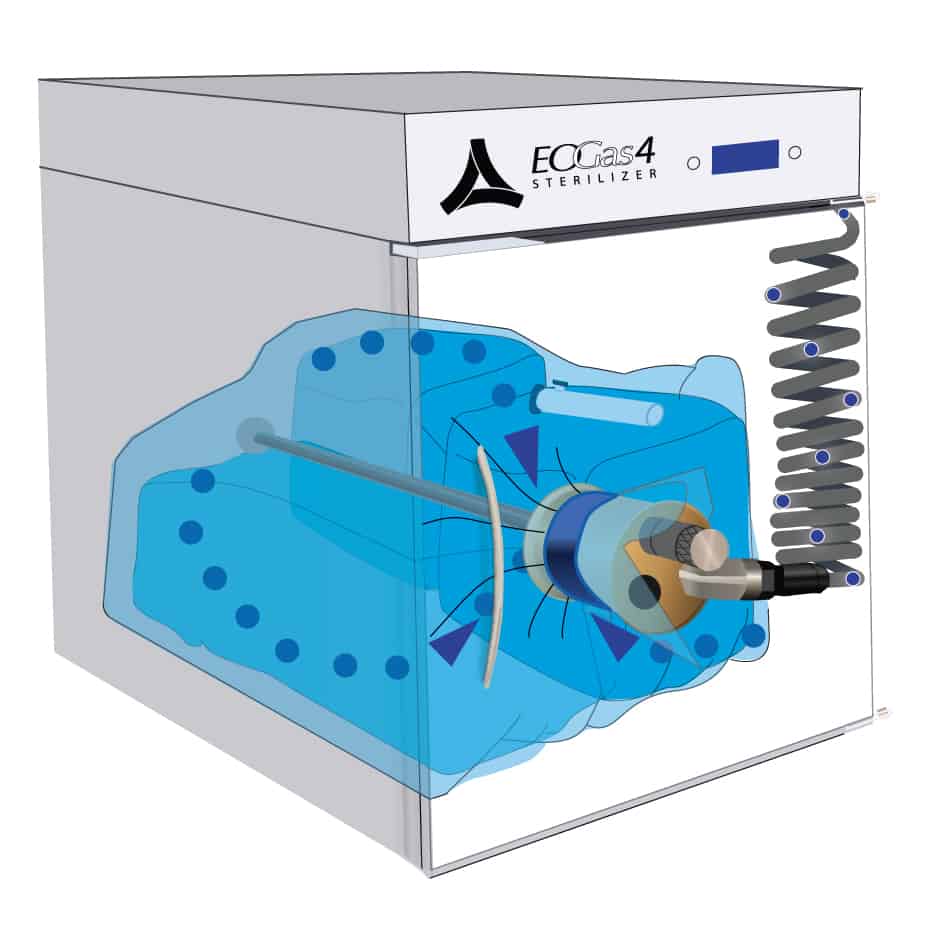
The sterilization cycle begins by purging excess air from the sterilization bag – the “Flexible Chamber.”
Air molecules, represented by the blue dots, are exhausted from the sterilization bag through the purge probe and purge tube.
With excess air removed, the ethylene oxide concentration in the sterilization bag is maximized during sterilization, thereby minimizing the amount of EO needed for effective sterilization.
Cartridge Activation & Sterilization
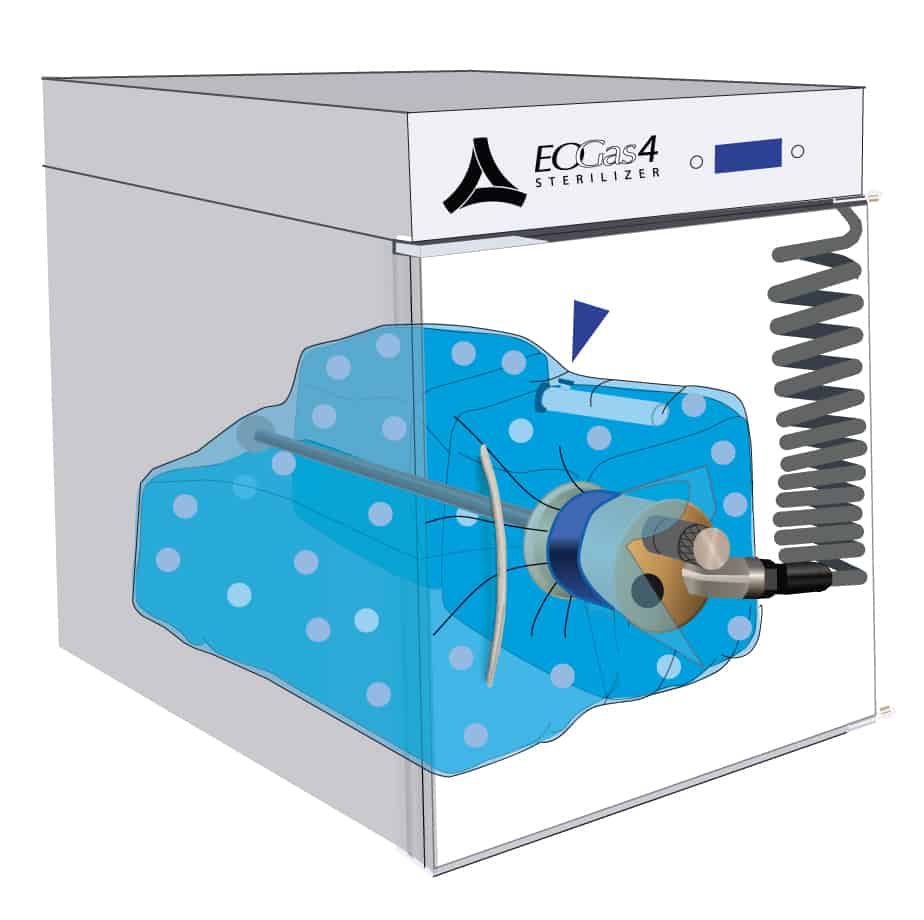
Once the initial purge is complete, the gas cartridge is manually activated by pressing the button on the cartridge through the wall of the sterilization bag.
Ethylene oxide, represented by the purple dots, spreads throughout the sterilization bag and permeates the items being processed. The gas-impermeable flexible chamber maintains a high gas concentration during the sterilization cycle.
Gas Ventilation
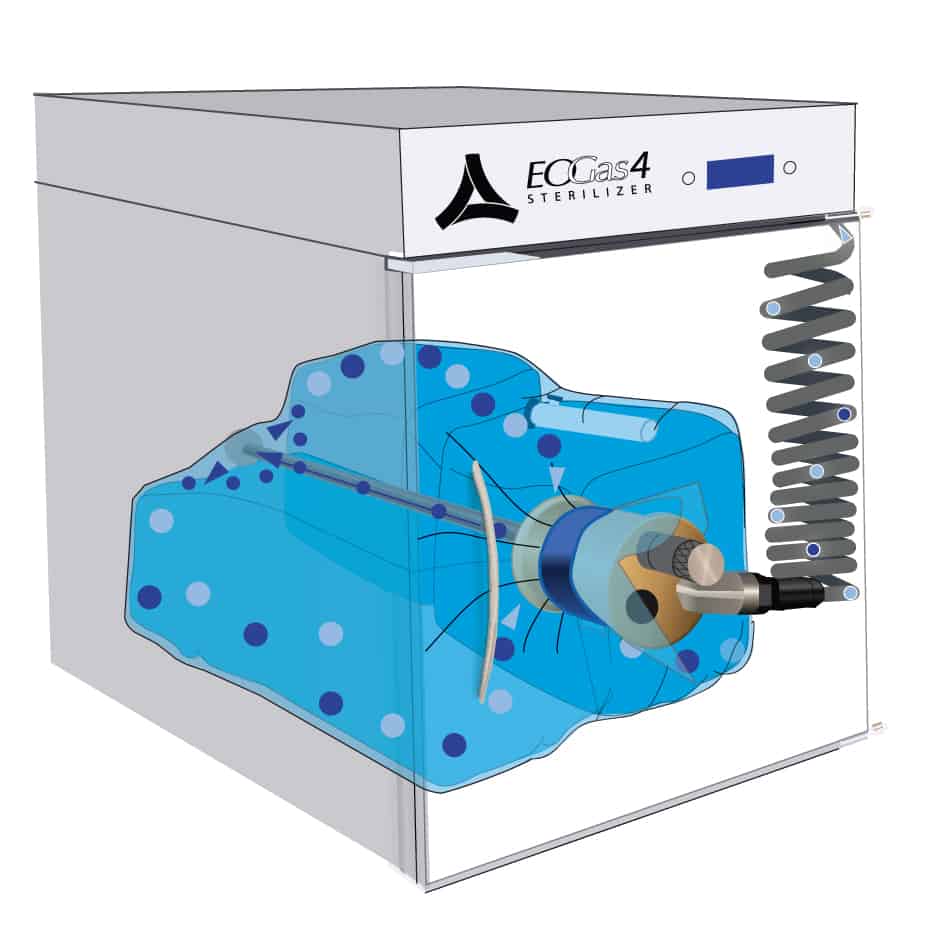
At the end of the sterilization cycle, the ventilation portion of the sterilization bag begins; 30 minutes ventilation after the 3 hours gas exposure and one hour after the 6 hour gas exposure. A valve in the purge probe opens allowing fresh air, represented by the blue dots, to travel down the probe and into the back of the bag. Ethylene oxide, represented by the purple dots, is exhausted through the purge probe.
Active Aeration
Conclusion
Aeration continues after ventilation, flushing air through the sterilization bag and out the purge tube. Aeration of the devices in the sterilization bag ends when the operator exits the cycle to remove the sterilization bag from the sterilizer.
Andersen’s breakthrough process is not that of yesteryear — this is EO sterilization reimagined.
Benefits of EO-Flexible Chamber Technology:
- Low temperature
- No steam injection
- No deep vacuum (which can damage delicate medical instruments)
- Ultra-efficient use of sterilant
- 90% less gas per cycle than competitors
- low emissions (zero emissions when paired with an abator)
These improvements create a zero-damage terminal sterilization process that has been exhaustively tested and FDA-cleared. Click Contact Us below to learn more.
Spread the Word
Expert Guidance for Your Needs
Tell us about your sterilization requirements — our experts are here to guide you to the perfect EO solution.

