Never has peace of mind required so little investment.
Andersen’s breakthrough technology is revolutionizing ethylene oxide (EO) sterilization. Equipped with flexible chamber technology, the newly released EOGas 4PLUS requires a fraction of the amount of ethylene oxide used by its rigid-chamber competitors. This modern-day approach to gas sterilization not only enhances efficiency but also ensures a gentler sterilization process at lower temperatures. Let’s take a look at how this breakthrough technology works.
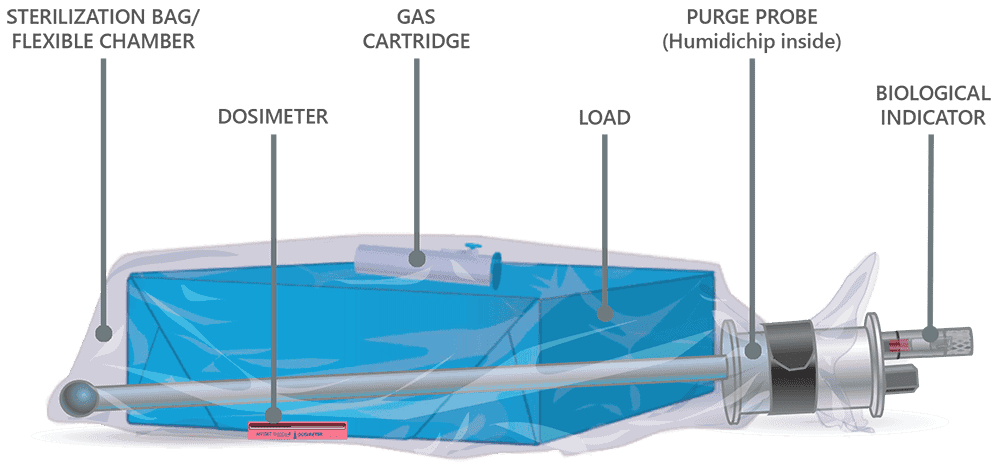
How EO-FCT works
Andersen’s proprietary EO-Flexible Chamber Technology process is a unique sterilization method. The graphic below illustrates a loaded sterilization bag or flexible chamber secured with a hook-and-loop strap around a purge probe.
Air Purge
The sterilization cycle begins by purging excess air from the sterilization bag, the flexible chamber. Air molecules are exhausted from the sterilization bag through the purge probe and purge tube. With excess air removed, the ethylene oxide concentration in the sterilization bag is maximized during sterilization, thereby minimizing the amount of EO needed for effective sterilization.
Cartridge Activation and Sterilization
Once the initial purge is complete, the gas cartridge is manually activated by pressing the button on the cartridge through the wall of the sterilization bag. Ethylene oxide spreads throughout the sterilization bag and permeates the devices being processed. The gas-impermeable flexible chamber maintains a high gas concentration during the sterilization cycle.
Gas Ventilation and Aeration Cycles
At the end of the sterilization cycle, the ventilation portion begins; 30 minutes of ventilation after the three-hour gas exposure and one hour after the six-hour gas exposure. A valve in the purge probe opens, allowing fresh air to travel down the probe and into the back of the bag. Ethylene oxide is exhausted through the purge probe.
Aeration continues after ventilation, flushing air through the sterilization bag and out the purge tube. Aeration of the devices in the sterilization bag ends when the operator exits the cycle to remove the sterilization bag from the sterilizer.
With FDA-cleared EOGas 4PLUS there is no need to culture endoscopes. The state-of-the-art system sterilizes and aerates in the same chamber and is the only system FDA-cleared for the terminal sterilization of long lumens — in just a few hours:
- Six-hour EO exposure for two duodenoscopes or colonoscopes or one of each
- One-hour ventilation
- Length of aeration follows manufacturer’s instructions for use (IFU)
- Four-hour rapid-release BI incubation
EOGas 4PLUS is preferred for the reprocessing of complex medical devices and long lumens that other modalities damage and degrade. The system goes above and beyond, offering a low per-cycle cost, space-saving tabletop design and unparalleled compatibility. When EOGas 4PLUS is paired with an abator, the system releases less than 0.02 grams of EO into the environment per cycle, making EOGas 4PLUS one of the most environmentally friendly systems on the market. EOGas 4PLUS — Low temperature. Low dose. Low emissions. Never has peace of mind required so little investment. Learn more about EOGas 4PLUS.
Spread the Word
Expert Guidance for Your Needs
Tell us about your sterilization requirements — our experts are here to guide you to the perfect EO solution.
