EO Sterilization: The FDA-Backed Method for Endoscope Reprocessing
Scopes May Pose A Deadly Risk
“Superbugs” are strains of bacteria, viruses, parasites, and fungi that are resistant to most antibiotics and other medications commonly used to treat infections. They include Carbapenem-resistant Enterobacteriaceae (CRE), which is a group of bacteria that is resistant to carbapenems, or highly potent antibiotics considered to be last-resort. CRE and similar organisms are linked to high mortality rates, particularly in patients with bloodstream infections, where mortality can reach 50% or more.1
Before 2015, infections linked to contaminated flexible endoscopes, specifically duodenoscopes, were attributed to failures in disinfection procedures. Reprocessing in accordance with manufacturer’s instructions had been associated with the prevention of cross-infection in most documented cases.2
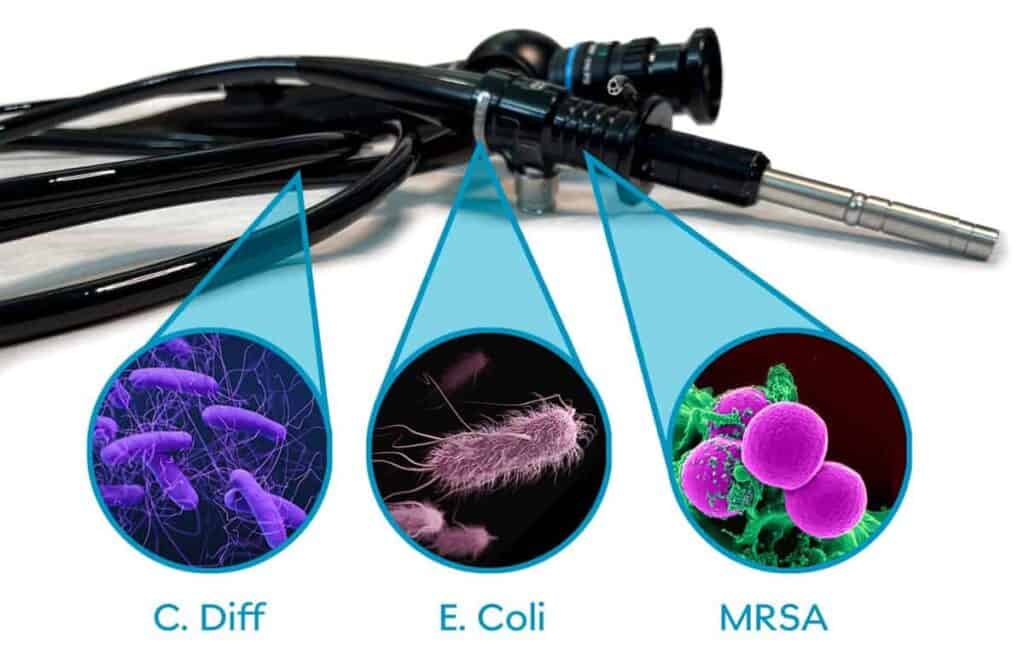
But in February of 2015, the FDA found that the complex physical design of duodenoscopes “may impede effective reprocessing,” and that “meticulously cleaning duodenoscopes prior to high-level disinfection should reduce the risk of transmitting infection but may not entirely eliminate all pathogens or spores.” 2
In August of 2015, the FDA published a safety communication advising healthcare facilities to consider implementing one or more of four “Supplemental Measures to Enhance Duodenoscope Reprocessing” and to reduce infection risk. Ethylene Oxide (EO) was included as one of these four measures.3
In the communication, the FDA made the distinction between disinfection and sterilization, stating that “duodenoscopes should be subjected to high-level disinfection following manual cleaning after each use. When possible and practical, duodenoscopes should be sterilized due to the greater margin of safety provided by sterilization.”3 Field evaluations later confirmed that ethylene oxide (EO) sterilization was the most effective supplemental measure, with validation studies showing it was the only method that ensured complete eradication of highly resistant microorganisms.4
Is High-Level Disinfection Enough?
Recent FDA alerts have expanded the focus beyond duodenoscopes to include bronchoscopes and urological endoscopes, which also carry infection risks. These trends highlight that the risk of infection associated with the use of duodenoscopes and other flexible scopes remains a concern. Healthcare facilities worldwide continue to report hospital-acquired infections (HAIs) resulting from inadequately cleaned devices, following manufacturers’ instructions and standard of care. These adverse events put our most vulnerable patients at risk. Deadly pathogens remaining on devices jeopardize the welfare of our young, our seniors and our immunocompromised patients.
Andersen Provides the Solution
In response to growing concerns about infections linked to reprocessed duodenoscopes, Andersen Sterilizers has worked closely with the FDA to develop and meet a much higher sterilization standard using ethylene oxide (EO). After years of collaboration and rigorous testing, Andersen successfully validated EO sterilization for duodenoscopes, colonoscopes, and other endoscopes at 10-6 SAL. Today, we are the ONLY company with FDA clearance for terminal sterilization of scopes with lumens as long as 13 feet and as narrow as 1.0 mm. Our modern EO systems are safe, efficient, and gentle on delicate instruments — offering a reliable solution to help healthcare providers reduce the risk of transmitting deadly superbugs like CRE. Ethylene oxide sterilization is the FDA-recommended method for reprocessing flexible endoscopes because of its greater margin of safety. We’re proud to be part of the solution.


Footnotes
1 October 16, 2014 “CRE infections of the bloodstream are reportedly associated with a mortality rate of as high as 40%-50%” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4198391/pdf/WJGE-6-457.pdf
3 August. 11, 2015 “Supplemental Measures to Enhance Duodenoscope Reprocessing: FDA Safety Communication” https://lfm-hcs.com/alerts/FDA_4_Supp_Mea sures.pdf
2 February 19, 2015 “Design of Endoscopic Retrograde Cholangiopancreatography (ERCP) Duodenoscopes May Impede Effective Cleaning: FDA Safety Communication” https://www.lfm-hcs.com/alerts/FDA_duodeno_Feb_2015.pdf
4 Feb. 4, 2019 “Use of Ethylene-Oxide Gas Sterilisation
to Terminate Multidrug-Resistant Bacterial Outbreaks
Linked to Duodenoscopes” https://bmjopengastro.bmj.com/content/bmjgast/6/1/e000282.full.pdf
Is it time to elevate your sterilization standards for safety and compliance?

Understanding Andersen’s FDA Clearances for Reliable Sterilization Solutions
Andersen Sterilizers are FDA cleared, ensuring the highest safety standards for your practice.
Discover the Benefits of Choosing Andersen Sterilizers for Your Facility
Our advanced technology not only meets but exceeds industry standards, providing peace of mind.

Request a Quote for Customized Sterilization Solutions Tailored to Your Needs
Get in touch with us today to receive a personalized quote for your facility.
Expert Guidance for Your Needs
Tell us about your sterilization requirements — our experts are here to guide you to the perfect EO solution.
