
In January, a CBS affiliate in Missouri reported that healthcare workers at Barnes-Jewish Hospital, one of the region’s largest healthcare systems, were raising concerns about potentially contaminated surgical instruments. Becker’s Hospital Review reported that the St. Louis-based hospital subsequently canceled, delayed and transferred multiple surgeries as a result of the sterile processing issues.
According to a recent study, an average of 3.5 defects were reported, with about 90% of SPDs reporting at least one measured defect in the week prior to the survey.1 The same study revealed that nearly 17% of defects were first identified in operating rooms, posing surgical safety risks and process delays. Recent research also shows that in 2025, approximately 2-5% of all surgical patients experienced surgical site infections (SSI), many due to improper instrument sterilization.2
“These hospital-acquired infections do not have to occur,” shared William Andersen, M.D., an orthopedic surgeon in central North Carolina and also CEO of Andersen Sterilizers. “Andersen’s FDA-cleared gas sterilizers are compact, efficient and effective while also protecting delicate, complex medical instruments. In fact, our EOGas 4PLUS sterilizer is the only system in the world cleared by FDA to terminally sterilize the longest duodenoscopes and colonoscopes (≥ 1.2 mm ID, ≤ 3530 mm maximum length of any channel).”
Designed and manufactured specifically for healthcare facilities, the EOGas 4PLUS system was introduced in 2025. The state-of-the-art system features award-winning EO-Flexible Chamber Technology, which enables the low-temperature system to achieve terminal sterilization (SAL 10-6) using just a microdose of ethylene oxide (EO) per exposure cycle. At just 17.6 grams per cycle, that’s 90% less EO than any other similar system on the market.
“EOGas 4PLUS is the perfect complement to any SPD’s sterile processing strategy,” continued Andersen, who is the son of the company’s founder, H.W. Andersen, M.D. “When processing endoscopes using EOGas 4PLUS, there is no need to culture. What’s more, this system sterilizes and aerates in the same chamber. Terminally sterilized medical devices can remain sterile for up to six months after processing.”
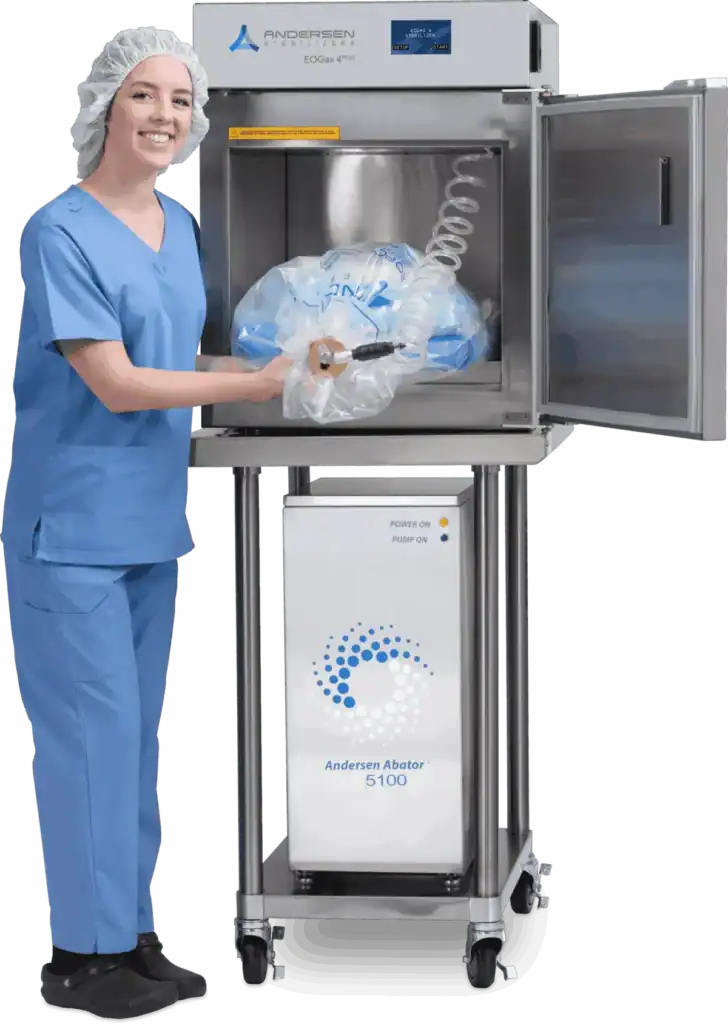
Unlike rigid-chamber systems, EO-Flexible Chamber Technology removes excess air from the sterilization bag (flexible chamber), allowing for a significantly smaller dose of EO. The purge probe, which is inserted into the sterilization bag when loading medical devices, allows fresh air to enter the bag and EO to evacuate the bag during ventilation and aeration.
Andersen’s environmentally friendly systems have low emissions. However, when paired with an Andersen abator, the sterilization systems release less than 0.02 grams of EO into the environment, making EOGas 4PLUS — and Andersen’s other FDA-cleared systems — some of the most environmentally friendly systems on the market.
EOGas 4PLUS features and capabilities include:
- Six-hour gas exposure for two duodenoscopes or colonoscopes or one of each
- One-hour ventilation
- Length of aeration follows manufacturer’s instructions for use (IFU)
- Four-hour rapid release BI
Lethal enough to kill deadly pathogens, EOGas 4PLUS is gentle enough to save complex medical devices, ensuring ongoing use time and again. Join healthcare facilities around the world adding EOGas 4PLUS to their SPD’s line of defense. Learn more at sterility.com.
- “Breaking the seal: Defects in sterile processing,” published in ScienceDirect (specifically in the American Journal of Infection Control) with a publication date of October 2025.
- “Unseen but Essential: The Sterile Processing Knowledge Demand and Staffing Crisis,” published in Surgical Directions, by Barbara McClenathanSeptember 9, 2025.
Spread the Word
Expert Guidance for Your Needs
Tell us about your sterilization requirements — our experts are here to guide you to the perfect EO solution.
