
Andersen’s FDA 510(K) Clearances
A 510(k) is a premarket submission made to FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent, to a legally marketed device.
FDA-Clearance
FDA clearances are crucial for sterilization systems, as they ensure patient safety by verifying that a system is both safe and effective in killing harmful microorganisms. Without FDA oversight, there would be no regulatory assurance that medical devices are properly sterilized, which could lead to life-threatening infections and other complications.
FDA Clearances & Patient and Public Health Protection
Addressing high-risk devices: High-risk devices, such as duodenoscopes, require rigorous testing to ensure that their complex designs can be effectively sterilized. FDA clearance shows that the manufacturer has met this “high bar” set by the agency.
Preventing infections: Improperly sterilized medical equipment, such as surgical instruments or implantable devices, can transmit dangerous pathogens. FDA clearance assures that a sterilization system can consistently achieve the required level of sterility to prevent infections.
Standardized effectiveness: The FDA requires manufacturers to provide scientific evidence that their system performs as labeled. This validation confirms that the system can kill a specific number of microorganisms, ensuring consistency and effectiveness across all products processed.
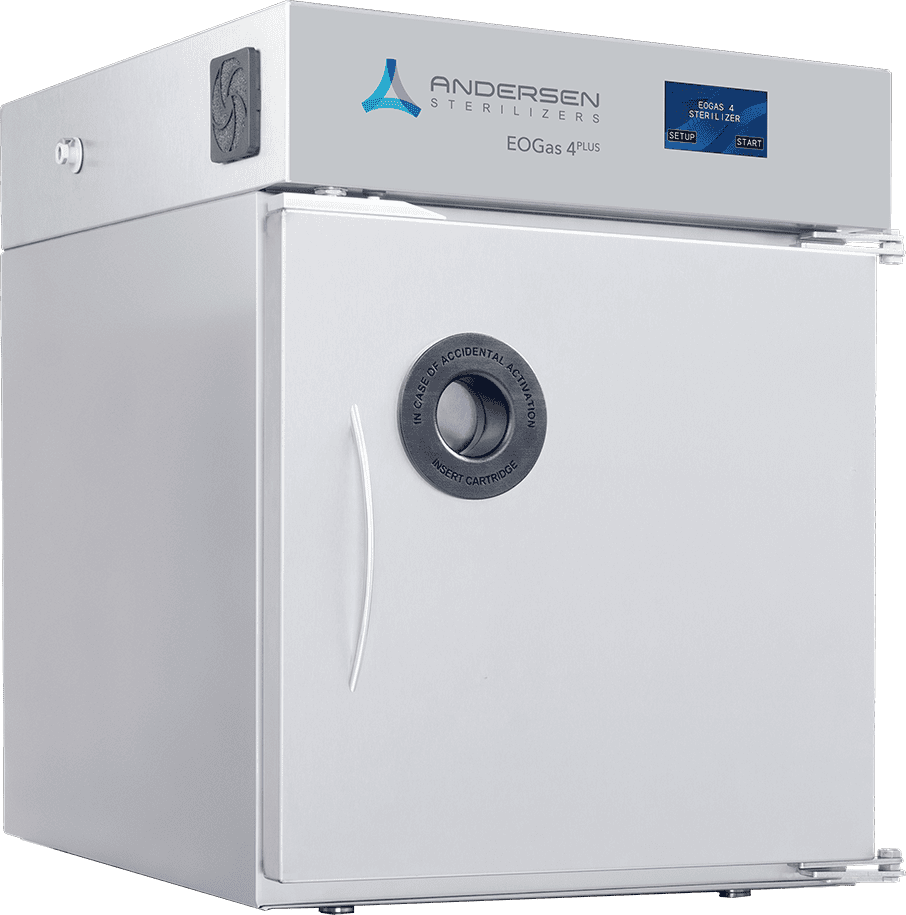
EOGas 4PLUS
Anprolene AN75 Series

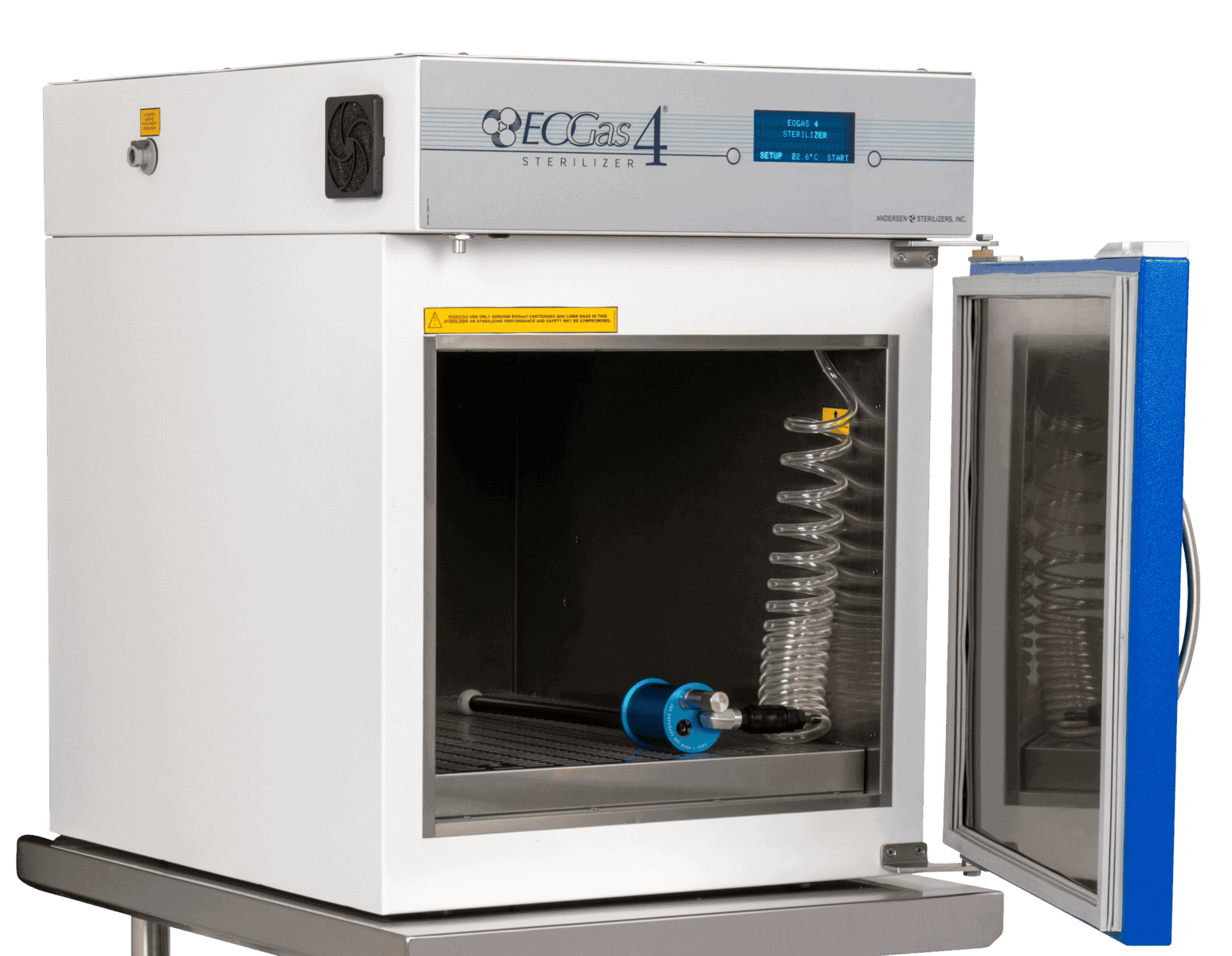
EOGas 4, 3- & 5-hour system
Expert Guidance for Your Needs
Tell us about your sterilization requirements — our experts are here to guide you to the perfect EO solution.
